Human Microdosing with Carcinogenic Polycyclic Aromatic Hydrocarbons: In Vivo Pharmacokinetics of Dibenzo[def,p]chrysene and Metabolites by UPLC Accelerator Mass Spectrometry
- PMID: 27494294
- PMCID: PMC5380438
- DOI: 10.1021/acs.chemrestox.6b00169
Human Microdosing with Carcinogenic Polycyclic Aromatic Hydrocarbons: In Vivo Pharmacokinetics of Dibenzo[def,p]chrysene and Metabolites by UPLC Accelerator Mass Spectrometry
Abstract
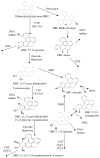
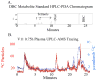
Metabolism is a key health risk factor following exposures to pro-carcinogenic polycyclic aromatic hydrocarbons (PAHs) such as dibenzo[def,p]chrysene (DBC), an IARC classified 2A probable human carcinogen. Human exposure to PAHs occurs primarily from the diet in nonsmokers. However, little data is available on the metabolism and pharmacokinetics in humans of high molecular weight PAHs (≥4 aromatic rings), including DBC. We previously determined the pharmacokinetics of DBC in human volunteers orally administered a microdose (29 ng; 5 nCi) of [14C]-DBC by accelerator mass spectrometry (AMS) analysis of total [14C] in plasma and urine. In the current study, we utilized a novel "moving wire" interface between ultraperformance liquid chromatography (UPLC) and AMS to detect and quantify parent DBC and its major metabolites. The major [14C] product identified in plasma was unmetabolized [14C]-DBC itself (Cmax = 18.5 ±15.9 fg/mL, Tmax= 2.1 ± 1.0 h), whereas the major metabolite was identified as [14C]-(+/-)-DBC-11,12-diol (Cmax= 2.5 ±1.3 fg/mL, Tmax= 1.8 h). Several minor species of [14C]-DBC metabolites were also detected for which no reference standards were available. Free and conjugated metabolites were detected in urine with [14C]-(+/-)-DBC-11,12,13,14-tetraol isomers identified as the major metabolites, 56.3% of which were conjugated (Cmax= 35.8 ± 23.0 pg/pool, Tmax = 6-12 h pool). [14C]-DBC-11,12-diol, of which 97.5% was conjugated, was also identified in urine (Cmax = 29.4 ± 11.6 pg/pool, Tmax = 6-12 h pool). Parent [14C]-DBC was not detected in urine. This is the first data set to assess metabolite profiles and associated pharmacokinetics of a carcinogenic PAH in human volunteers at an environmentally relevant dose, providing the data necessary for translation of high dose animal models to humans for translation of environmental health risk assessment.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


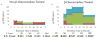
Similar articles
-
Human in Vivo Pharmacokinetics of [(14)C]Dibenzo[def,p]chrysene by Accelerator Mass Spectrometry Following Oral Microdosing.Chem Res Toxicol. 2015 Jan 20;28(1):126-34. doi: 10.1021/tx5003996. Epub 2014 Dec 10. Chem Res Toxicol. 2015. PMID: 25418912 Free PMC article. Clinical Trial.
-
Translating dosimetry of Dibenzo[def,p]chrysene (DBC) and metabolites across dose and species using physiologically based pharmacokinetic (PBPK) modeling.Toxicol Appl Pharmacol. 2022 Mar 1;438:115830. doi: 10.1016/j.taap.2021.115830. Epub 2021 Dec 18. Toxicol Appl Pharmacol. 2022. PMID: 34933053 Free PMC article.
-
In vitro metabolism of benzo[a]pyrene and dibenzo[def,p]chrysene in rodent and human hepatic microsomes.Toxicol Lett. 2014 Jul 3;228(1):48-55. doi: 10.1016/j.toxlet.2014.04.004. Epub 2014 Apr 21. Toxicol Lett. 2014. PMID: 24769260 Free PMC article.
-
Practical experience of using human microdosing with AMS analysis to obtain early human drug metabolism and PK data.Bioanalysis. 2010 Mar;2(3):429-40. doi: 10.4155/bio.10.6. Bioanalysis. 2010. PMID: 21083253 Review.
-
Use of Accelerator Mass Spectrometry in Human Health and Molecular Toxicology.Chem Res Toxicol. 2016 Dec 19;29(12):1976-1986. doi: 10.1021/acs.chemrestox.6b00234. Epub 2016 Oct 11. Chem Res Toxicol. 2016. PMID: 27726383 Free PMC article. Review.
Cited by
-
Effects of Naphtho[2,1-a]pyrene Exposure on CYP1A1 Expression: An in Vivo and in Vitro Mechanistic Study Exploring the Role of Posttranscriptional Modification.Environ Health Perspect. 2024 Aug;132(8):87003. doi: 10.1289/EHP14055. Epub 2024 Aug 12. Environ Health Perspect. 2024. PMID: 39133094 Free PMC article.
-
Strategic, feasibility, economic, and cultural aspects of phase 0 approaches: Is it time to change the drug development process in order to increase productivity?Clin Transl Sci. 2022 Jun;15(6):1355-1379. doi: 10.1111/cts.13269. Epub 2022 Apr 21. Clin Transl Sci. 2022. PMID: 35278281 Free PMC article. Review.
-
Benzo[a]pyrene (BaP) metabolites predominant in human plasma following escalating oral micro-dosing with [14C]-BaP.Environ Int. 2022 Jan 15;159:107045. doi: 10.1016/j.envint.2021.107045. Epub 2021 Dec 15. Environ Int. 2022. PMID: 34920278 Free PMC article.
-
[Progress in the metabolic and biotransformation of polycyclic aromatic hydrocarbons and their derivatives in humans].Se Pu. 2025 Jun;43(6):571-584. doi: 10.3724/SP.J.1123.2024.11030. Se Pu. 2025. PMID: 40394737 Free PMC article. Review. Chinese.
-
HPLC-Parallel accelerator and molecular mass spectrometry analysis of 14C-labeled amino acids.J Chromatogr B Analyt Technol Biomed Life Sci. 2023 Feb 1;1216:123590. doi: 10.1016/j.jchromb.2022.123590. Epub 2023 Jan 4. J Chromatogr B Analyt Technol Biomed Life Sci. 2023. PMID: 36669256 Free PMC article.
References
-
- IARC. Polynuclear aromatic compounds, Part 1: chemical, environmental, and experimental data. World Health Organization International Agency For Research On Cancer; Lyon, France: 1983. Monographs on the Evaluation of Carcinogenic Risks to Humans. - PubMed
-
- Menzie CA, Potocki BB, Santodonato J. Exposure to carcinogenic PAHs in the environment. Environmn Sci Technol. 1992;26:1278–1284.
-
- Dennis MJ, Massey RC, Cripps G, Venn I, Howarth N, Lee G. Factors affecting the polycyclic aromatic hydrocarbon content of cereals, fats and other food-products. Food Addit Contam. 1991;8:517–539. - PubMed
-
- Jakszyn P, Agudo A, Albanez R, Garcia-Closas R, Pera G, Amiano P, Gonzalez CA. Development of a food database of nitrosamines, heterocyclic amines, and polycyclic aromatic hydrocarbons. J Nutr. 2004;134:2011–2014. - PubMed
-
- EPA; Code of Federal Regulations PART 423 - Steam electric power generating point of source category. E.P. Agency, editor. Title 40 - Protection of Environment. Chapter 1 - Environmental protection agency subchapter N - Effluent guidelines and standards. 2007
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

