Tankyrase Requires SAM Domain-Dependent Polymerization to Support Wnt-β-Catenin Signaling
- PMID: 27494558
- PMCID: PMC4980433
- DOI: 10.1016/j.molcel.2016.06.019
Tankyrase Requires SAM Domain-Dependent Polymerization to Support Wnt-β-Catenin Signaling
Abstract
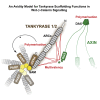
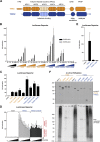
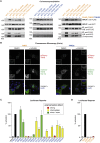
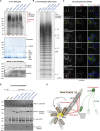
The poly(ADP-ribose) polymerase (PARP) Tankyrase (TNKS and TNKS2) is paramount to Wnt-β-catenin signaling and a promising therapeutic target in Wnt-dependent cancers. The pool of active β-catenin is normally limited by destruction complexes, whose assembly depends on the polymeric master scaffolding protein AXIN. Tankyrase, which poly(ADP-ribosyl)ates and thereby destabilizes AXIN, also can polymerize, but the relevance of these polymers has remained unclear. We report crystal structures of the polymerizing TNKS and TNKS2 sterile alpha motif (SAM) domains, revealing versatile head-to-tail interactions. Biochemical studies informed by these structures demonstrate that polymerization is required for Tankyrase to drive β-catenin-dependent transcription. We show that the polymeric state supports PARP activity and allows Tankyrase to effectively access destruction complexes through enabling avidity-dependent AXIN binding. This study provides an example for regulated signal transduction in non-membrane-enclosed compartments (signalosomes), and it points to novel potential strategies to inhibit Tankyrase function in oncogenic Wnt signaling.
Copyright © 2016 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Alvarez-Gonzalez R., Jacobson M.K. Characterization of polymers of adenosine diphosphate ribose generated in vitro and in vivo. Biochemistry. 1987;26:3218–3224. - PubMed
-
- Bienz M. Signalosome assembly by domains undergoing dynamic head-to-tail polymerization. Trends Biochem. Sci. 2014;39:487–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

