Combinatorial therapeutic targeting of BMP2 and MEK-ERK pathways in NF1-associated malignant peripheral nerve sheath tumors
- PMID: 27494873
- PMCID: PMC5302981
- DOI: 10.18632/oncotarget.11036
Combinatorial therapeutic targeting of BMP2 and MEK-ERK pathways in NF1-associated malignant peripheral nerve sheath tumors
Abstract
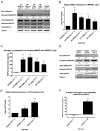
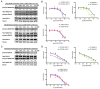
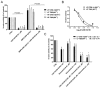
The clinical management of malignant peripheral nerve sheath tumors (MPNSTs) is challenging not only due to its aggressive and invasive nature, but also limited therapeutic options. Using gene expression profiling, our lab identified BMP2-SMAD1/5/8 pathway as a potential therapeutic target for treating MPNSTs. In this study, we explored the therapeutic impact of targeting BMP2-SMAD1/5/8 pathway in conjunction with RAS-MEK-ERK signaling, which is constitutively activated in MPNSTs. Our results indicated that single agent treatment with LDN-193189, a BMP2 Type I receptor inhibitor, did not affect the growth and survival of MPNST cells at biochemically relevant inhibitory concentrations. However, addition of a MEK1/2 inhibitor, selumetinib, to LDN-193189-treated cells resulted in significant inhibition of cell growth and induction of cell death. LDN-193189 at biochemically effective concentrations significantly inhibited motility and invasiveness of MPNST cells, and these effects were enhanced by the addition of selumetinib. Overall, our results advocate for a combinatorial therapeutic approach for MPNSTs that not only targets the growth and survival via inhibition of MEK1/2, but also its malignant spread by suppressing the activation of BMP2-SMAD1/5/8 pathway. Importantly, these studies were conducted in low-passage patient-derived MPNST cells, allowing for an investigation of the effects of the proposed drug treatments in a biologically-relevant context.
Keywords: BMP2; cell signaling; combinatorial targeted therapy; malignant peripheral nerve sheath tumors; neurofibromatosis type 1.
Conflict of interest statement
The authors do not have any potential conflicts of interest in regards to the submitted work.
Figures


References
-
- Friedman JM. Epidemiology of neurofibromatosis type 1. Am J Med Genet. 1999;89:1–6. - PubMed
-
- Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014;13:834–843. - PubMed
-
- Roma P. Letter to the Editor Mosaic (Segmental) Neurofibromatosis Type 1 (NF1) and Type 2 (NF2): No Longer Neurofibromatosis Type 5. 2001;180:178–180. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

