Reduced β-Cell Secretory Capacity in Pancreatic-Insufficient, but Not Pancreatic-Sufficient, Cystic Fibrosis Despite Normal Glucose Tolerance
- PMID: 27495225
- PMCID: PMC5204312
- DOI: 10.2337/db16-0394
Reduced β-Cell Secretory Capacity in Pancreatic-Insufficient, but Not Pancreatic-Sufficient, Cystic Fibrosis Despite Normal Glucose Tolerance
Abstract
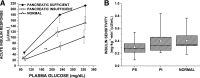
Patients with pancreatic-insufficient cystic fibrosis (PI-CF) are at increased risk for developing diabetes. We determined β-cell secretory capacity and insulin secretory rates from glucose-potentiated arginine and mixed-meal tolerance tests (MMTTs), respectively, in pancreatic-sufficient cystic fibrosis (PS-CF), PI-CF, and normal control subjects, all with normal glucose tolerance, in order to identify early pathophysiologic defects. Acute islet cell secretory responses were determined under fasting, 230 mg/dL, and 340 mg/dL hyperglycemia clamp conditions. PI-CF subjects had lower acute insulin, C-peptide, and glucagon responses compared with PS-CF and normal control subjects, indicating reduced β-cell secretory capacity and α-cell function. Fasting proinsulin-to-C-peptide and proinsulin secretory ratios during glucose potentiation were higher in PI-CF, suggesting impaired proinsulin processing. In the first 30 min of the MMTT, insulin secretion was lower in PI-CF compared with PS-CF and normal control subjects, and glucagon-like peptide 1 and gastric inhibitory polypeptide were lower compared with PS-CF, and after 180 min, glucose was higher in PI-CF compared with normal control subjects. These findings indicate that despite "normal" glucose tolerance, adolescents and adults with PI-CF have impairments in functional islet mass and associated early-phase insulin secretion, which with decreased incretin responses likely leads to the early development of postprandial hyperglycemia in CF.
© 2017 by the American Diabetes Association.
Figures


Comment in
-
Exocrine and Endocrine Interactions in Cystic Fibrosis: A Potential Key to Understanding Insulin Secretion in Health and Disease?Diabetes. 2017 Jan;66(1):20-22. doi: 10.2337/dbi16-0049. Diabetes. 2017. PMID: 27999103 Free PMC article. No abstract available.
References
-
- Marshall BC, Butler SM, Stoddard M, Moran AM, Liou TG, Morgan WJ. Epidemiology of cystic fibrosis-related diabetes. J Pediatr 2005;146:681–687 - PubMed
-
- Gudipaty, L, Rickels MR. Pancreatogenic (Type 3c) Diabetes. 2015. Pancreapedia: Exocrine Pancreas Knowledge Base. DOI: 10.3998/panc.2015.35
-
- Kuo P, Stevens JE, Russo A, et al. . Gastric emptying, incretin hormone secretion, and postprandial glycemia in cystic fibrosis--effects of pancreatic enzyme supplementation. J Clin Endocrinol Metab 2011;96:E851–E855 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

