CUB domain-containing protein 1 and the epidermal growth factor receptor cooperate to induce cell detachment
- PMID: 27495374
- PMCID: PMC4974783
- DOI: 10.1186/s13058-016-0741-1
CUB domain-containing protein 1 and the epidermal growth factor receptor cooperate to induce cell detachment
Abstract
Background: While localized malignancies often respond to available therapies, most disseminated cancers are refractory. Novel approaches, therefore, are needed for the treatment of metastatic disease. CUB domain-containing protein1 (CDCP1) plays an important role in metastasis and drug resistance; the mechanism however, is poorly understood.
Methods: Breast cancer cell lines were engineered to stably express EGFR, CDCP1 or phosphorylation site mutants of CDCP1. These cell lines were used for immunoblot analysis or affinity purification followed by immunoblot analysis to assess protein phosphorylation and/or protein complex formation with CDCP1. Kinase activity was evaluated using phosphorylation site-specific antibodies and immunoblot analysis in in vitro kinase assays. Protein band excision and mass spectrometry was utilized to further identify proteins complexed with CDCP1 or ΔCDCP1, which is a mimetic of the cleaved form of CDCP1. Cell detachment was assessed using cell counting.
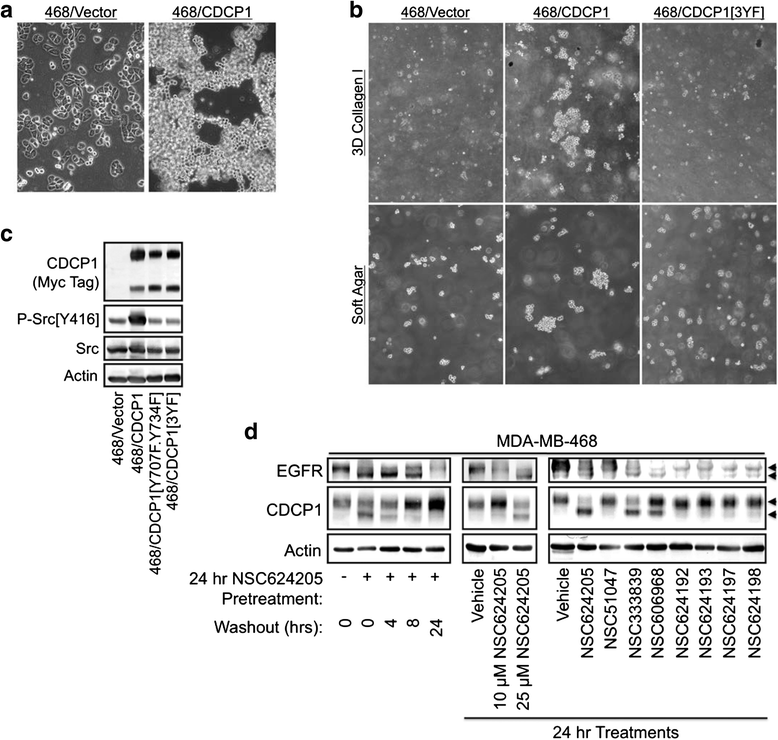
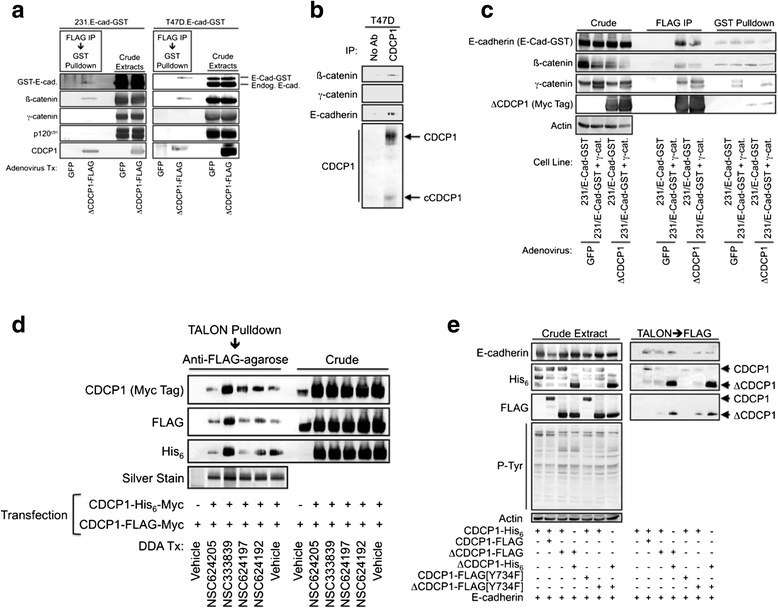
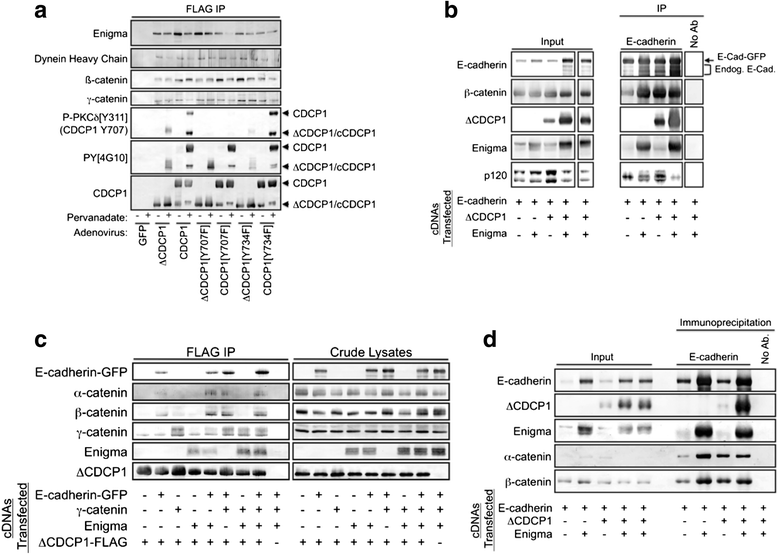
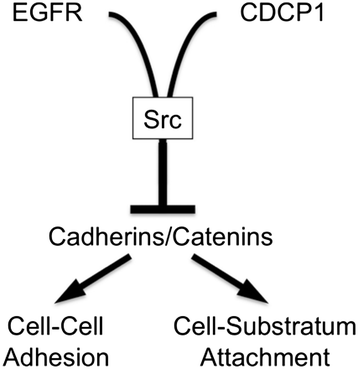
Results: This paper reports that CDCP1 forms ternary protein complexes with Src and EGFR, facilitating Src activation and Src-dependent EGFR transactivation. Importantly, we have discovered that a class of compounds termed Disulfide bond Disrupting Agents (DDAs) blocks CDCP1/EGFR/Src ternary complex formation and downstream signaling. CDCP1 and EGFR cooperate to induce detachment of breast cancer cells from the substratum and to disrupt adherens junctions. Analysis of CDCP1-containing complexes using proteomics techniques reveals that CDCP1 associates with several proteins involved in cell adhesion, including adherens junction and desmosomal cadherins, and cytoskeletal elements.
Conclusions: Together, these results suggest that CDCP1 may facilitate loss of adhesion by promoting activation of EGFR and Src at sites of cell-cell and cell-substratum contact.
Keywords: Adhesion; Breast cancer; CDCP1; E-cadherin; EGFR; Src.
Figures





References
-
- Brown TA, Yang TM, Zaitsevskaia T, Xia Y, Dunn CA, Sigle RO, Knudsen B, Carter WG. Adhesion or plasmin regulates tyrosine phosphorylation of a novel membrane glycoprotein p80/gp140/CUB domain-containing protein 1 in epithelia. J Biol Chem. 2004;279:14772–83. doi: 10.1074/jbc.M309678200. - DOI - PubMed
-
- Gioia R, Leroy C, Drullion C, Lagarde V, Etienne G, Dulucq S, Lippert E, Roche S, Mahon FX, Pasquet JM. Quantitative phosphoproteomics revealed interplay between Syk and Lyn in the resistance to nilotinib in chronic myeloid leukemia cells. Blood. 2011;118:2211–21. doi: 10.1182/blood-2010-10-313692. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

