Safety and Efficacy of Alemtuzumab Induction in Highly Sensitized Pediatric Renal Transplant Recipients
- PMID: 27495773
- PMCID: PMC7228615
- DOI: 10.1097/TP.0000000000001416
Safety and Efficacy of Alemtuzumab Induction in Highly Sensitized Pediatric Renal Transplant Recipients
Abstract
Background: Studies show that alemtuzumab, a potent lymphocyte-depleting agent, is well tolerated in pediatric renal transplantation. We report on the use of alemtuzumab induction in highly HLA sensitized (HS) pediatric kidney transplant patients.
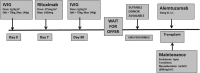
Methods: Fifty pediatric renal transplants were performed from 1/2009-12/2014. 15 HS patients received IVIG (2 g/kg ×2 doses)/rituximab (375 mg/m ×1) for desensitization with alemtuzumab induction (15-30 mg, 1 dose, subcutaneous), whereas 35 nonsensitized patients received anti-IL-2R. Graft survival and infections were compared between 2 groups.
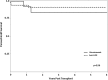
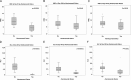
Results: All HS patients had received a prior transplant and were older with lower risk for viral infections due to serostatus. Patient survival was 100%, and graft outcomes were similar with mean 1-year creatinine of 1.03 ± 0.45 versus 0.99 ± 0.6 (P = 0.48). Although a higher incidence of acute cellular rejection was seen in HS patients receiving alemtuzumab (P = 0.001), there was a nonsignificant difference in antibody-mediated rejection. White blood cell and absolute lymphocyte count were significantly lower in alemtuzumab group at 30 days (P < 0.0001) and at 1 year (P = 0.026 and P = 0.001), respectively. There was no significant difference in bacterial, viral, or fungal infections after transplant.
Conclusions: Alemtuzumab induction with desensitization led to nearly equivalent graft survival and functional outcomes in HS pediatric patients as nonsensitized patients receiving anti-IL-2R induction. With this small sample size, we observed significant reduction of white blood cell and absolute lymphocyte count up to 1 year posttransplant. The risk of infection was comparable between the 2 groups; however, patients who received alemtuzumab were older and at lower risk of viral infection due to serostatus.
Conflict of interest statement
The authors declare no funding or conflicts of interest.
Figures

References
-
- Shapiro R, Sarwal MM. Pediatric kidney transplantation Pediatr Clin North Am 2010. 57393–400 - PubMed
-
- Vo AA, Lukovsky M, Toyoda M. Rituximab and intravenous immune globulin for desensitization during renal transplantation N Engl J Med 2008. 359242–251 - PubMed
-
- Vo AA, Sinha A, Haas M. Factors predicting risk for antibody-mediated rejection and graft loss in highly human leukocyte antigen sensitized patients transplanted after desensitization Transplantation 2015. 991423–1430 - PubMed
-
- Montgomery RA, Lonze BE, King KE. Desensitization in HLA-incompatible kidney recipients and survival N Engl J Med 2011. 365318–326 - PubMed
-
- Vo AA, Peng A, Toyoda M. Use of intravenous immune globulin and rituximab for desensitization of highly HLA-sensitized patients awaiting kidney transplantation Transplantation 2010. 891095–1102 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

