Preventing and Managing Toxicities of High-Dose Methotrexate
- PMID: 27496039
- PMCID: PMC5153332
- DOI: 10.1634/theoncologist.2015-0164
Preventing and Managing Toxicities of High-Dose Methotrexate
Abstract
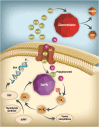
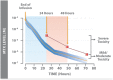
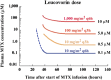
: High-dose methotrexate (HDMTX), defined as a dose higher than 500 mg/m2, is used to treat a range of adult and childhood cancers. Although HDMTX is safely administered to most patients, it can cause significant toxicity, including acute kidney injury (AKI) in 2%-12% of patients. Nephrotoxicity results from crystallization of methotrexate in the renal tubular lumen, leading to tubular toxicity. AKI and other toxicities of high-dose methotrexate can lead to significant morbidity, treatment delays, and diminished renal function. Risk factors for methotrexate-associated toxicity include a history of renal dysfunction, volume depletion, acidic urine, and drug interactions. Renal toxicity leads to impaired methotrexate clearance and prolonged exposure to toxic concentrations, which further worsen renal function and exacerbate nonrenal adverse events, including myelosuppression, mucositis, dermatologic toxicity, and hepatotoxicity. Serum creatinine, urine output, and serum methotrexate concentration are monitored to assess renal clearance, with concurrent hydration, urinary alkalinization, and leucovorin rescue to prevent and mitigate AKI and subsequent toxicity. When delayed methotrexate excretion or AKI occurs despite preventive strategies, increased hydration, high-dose leucovorin, and glucarpidase are usually sufficient to allow renal recovery without the need for dialysis. Prompt recognition and effective treatment of AKI and associated toxicities mitigate further toxicity, facilitate renal recovery, and permit patients to receive other chemotherapy or resume HDMTX therapy when additional courses are indicated.
Implications for practice: High-dose methotrexate (HDMTX), defined as a dose higher than 500 mg/m2, is used for a range of cancers. Although HDMTX is safely administered to most patients, it can cause significant toxicity, including acute kidney injury (AKI), attributable to crystallization of methotrexate in the renal tubular lumen, leading to tubular toxicity. When AKI occurs despite preventive strategies, increased hydration, high-dose leucovorin, and glucarpidase allow renal recovery without the need for dialysis. This article, based on a review of the current associated literature, provides comprehensive recommendations for prevention of toxicity and, when necessary, detailed treatment guidance to mitigate AKI and subsequent toxicity.
Keywords: Acute kidney injury; Glucarpidase; High-dose methotrexate; Leucovorin; Methotrexate; Pharmacokinetics.
©AlphaMed Press.
Conflict of interest statement
of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. The Oncologist. 2006;11:694–703. - PubMed
-
- Stoller RG, Hande KR, Jacobs SA, et al. Use of plasma pharmacokinetics to predict and prevent methotrexate toxicity. N Engl J Med. 1977;297:630–634. - PubMed
-
- Mitchell MS, Wawro NW, DeConti RC, et al. Effectiveness of high-dose infusions of methotrexate followed by leucovorin in carcinoma of the head and neck. Cancer Res. 1968;28:1088–1094. - PubMed
-
- Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol. 1998;16:859–863. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

