Efficacy and safety of sucroferric oxyhydroxide compared with sevelamer hydrochloride in Japanese haemodialysis patients with hyperphosphataemia: A randomized, open-label, multicentre, 12-week phase III study
- PMID: 27496336
- PMCID: PMC5347921
- DOI: 10.1111/nep.12891
Efficacy and safety of sucroferric oxyhydroxide compared with sevelamer hydrochloride in Japanese haemodialysis patients with hyperphosphataemia: A randomized, open-label, multicentre, 12-week phase III study
Abstract
Aim: We aimed to investigate the non-inferiority of PA21 (sucroferric oxyhydroxide) to sevelamer hydrochloride (sevelamer) in terms of efficacy and safety in Japanese haemodialysis patients with hyperphosphataemia.
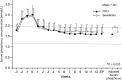
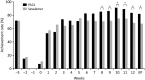
Methods: In this Phase III, open-label, multicentre study, 213 haemodialysis patients with hyperphosphataemia were randomized to PA21 or sevelamer treatment for 12 weeks. The primary outcome was adjusted serum phosphorus concentration at the end of treatment; the non-inferiority of PA21 was confirmed if the upper limit of the two-sided 95% confidence interval (CI) is ≤0.32 mmol/L. Secondary outcomes were corrected serum calcium and intact-parathyroid hormone concentrations. Adverse events (AEs) and adverse drug reactions (ADRs) were evaluated.
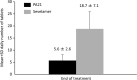
Results: The adjusted mean serum phosphorus concentration at the end of treatment confirmed the non-inferiority of PA21 for lowering serum phosphorus compared with sevelamer (1.62 vs 1.72 mmol/L; difference, -0.11 mmol/L; 95% CI, -0.20 to -0.02 mmol/L). The mean daily tablet intake was 5.6 ± 2.6 and 18.7 ± 7.1 tablets in the PA21 and sevelamer groups, respectively. The incidences of AEs and ADRs were not significantly different between the two groups.
Conclusion: The non-inferiority of PA21 to sevelamer was confirmed for the treatment of Japanese haemodialysis patients with hyperphosphataemia. PA21 was effective, safe, and well tolerated, while having a considerably lower pill burden than sevelamer.
Keywords: PA21 compound; haemodialysis; hyperphosphataemia; sevelamer; sucroferric oxyhydroxide.
© 2016 The Authors Nephrology published by John Wiley & Sons Australia, Ltd on behalf of Asian Pacific Society of Nephrology.
Figures

References
-
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J. Am. Soc. Nephrol. 2004; 15: 2208–2218. - PubMed
-
- Kalantar‐Zadeh K, Kuwae N, Regidor DL et al. Survival predictability of time‐varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006; 70: 771–780. - PubMed
-
- Fukagawa M, Yokoyama K, Koiwa F et al. Clinical practice guideline for the management of chronic kidney disease‐mineral and bone disorder. Ther. Apher. Dial. 2013; 17: 247–288. - PubMed
-
- National Kidney Foundation . K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am. J. Kidney Dis. 2003; 42: S1–S201. - PubMed
-
- Bleyer AJ, Burke SK, Dillon M et al. A comparison of the calcium‐free phosphate binder sevelamer hydrochloride with calcium acetate in the treatment of hyperphosphatemia in hemodialysis patients. Am. J. Kidney Dis. 1999; 33: 694–701. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

