Relationship between Overall Survival and Response or Progression-Free Survival in Advanced Non-Small Cell Lung Cancer Patients Treated with Anti-PD-1/PD-L1 Antibodies
- PMID: 27496650
- PMCID: PMC7086375
- DOI: 10.1016/j.jtho.2016.07.017
Relationship between Overall Survival and Response or Progression-Free Survival in Advanced Non-Small Cell Lung Cancer Patients Treated with Anti-PD-1/PD-L1 Antibodies
Abstract
Introduction: Alternative predictive end points for overall survival (OS), such as tumor response and progression-free survival (PFS), are useful in the early detection of drug efficacy; however, they have not been fully investigated in patients with advanced NSCLC treated with anti-programmed death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) antibodies.
Methods: In a systematic review of the reported prospective clinical trials, data for response rate, median PFS, and median OS were extracted from 12 arms in 10 reported clinical trials using anti-PD-1/PD-L1 antibody, and their correlation was investigated. In a retrospective analysis at our institution, OS was compared according to tumor response on 5- to 9-week computed tomography scans and status of being progression-free at 8, 16, and 24 weeks by landmark analysis in 71 patients with advanced NSCLC treated with anti-PD-1/PD-L1 antibodies between 2013 and 2015.
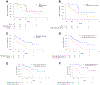
Results: In a systematic review, moderate correlations between median OS and median PFS (p = 0.120, r = 0.473) and between median OS and response rate (p = 0.141, r = 0.452) were identified using the Spearman correlation coefficient, although these correlations were not statistically significant. In a retrospective analysis of patients treated at our institution, disease control (partial response [PR]/stable disease versus progressive disease/not evaluable), and progression-free status at 8, 16, and 24 weeks significantly predicted OS (Cox proportional hazards model, PR/stable disease versus progressive disease/not evaluable, p = 0.0104, HR = 3.041; 8-week progression-free yes versus no, p = 0.0183, HR = 2.684; 16-week progression-free yes versus no, p = 0.0036, HR = 4.009; and 24-week progression-free yes versus no, p = 0.0002, HR = 12.726).
Conclusions: Both disease control (PR plus stable disease status) and landmark progression-free survival were correlated with OS, with the longer interval landmark PFS being the best predictor of survival in patients with NSCLC treated with anti-PD-1/PD-L1 antibodies.
Keywords: Alternative end point; Anti–PD-1 antibody; Anti–PD-L1 antibody; Immune checkpoint inhibitor; Non–small cell lung cancer; Overall survival.
Copyright © 2016 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Figures

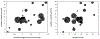
References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Peters S, Adjei AA, Gridelli C, et al. Metastatic nonsmall-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii56–vii64. - PubMed
-
- National comprehensive cancer network. NCCN clinical practice guidelines in oncology (NCCN Guidelines) Version 2. 2016. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed December 1, 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

