How have research questions and methods used in clinical trials published in Clinical Rehabilitation changed over the last 30 years?
- PMID: 27496695
- PMCID: PMC4976657
- DOI: 10.1177/0269215516658939
How have research questions and methods used in clinical trials published in Clinical Rehabilitation changed over the last 30 years?
Abstract
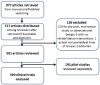
Research in rehabilitation has grown from a rare phenomenon to a mature science and clinical trials are now common. The purpose of this study is to estimate the extent to which questions posed and methods applied in clinical trials published in Clinical Rehabilitation have evolved over three decades with respect to accepted standards of scientific rigour. Studies were identified by journal, database, and hand searching for the years 1986 to 2016.A total of 390 articles whose titles suggested a clinical trial of an intervention, with or without randomization to form groups, were reviewed. Questions often still focused on methods to be used (57%) rather than what knowledge was to be gained. Less than half (43%) of the studies delineated between primary and secondary outcomes; multiple outcomes were common; and sample sizes were relatively small (mean 83, range 5 to 3312). Blinding of assessors was common (72%); blinding of study subjects was rare (19%). In less than one-third of studies was intention-to-treat analysis done correctly; power was reported in 43%. There is evidence of publication bias as 83% of studies reported either a between-group or a within-group effect. Over time, there was an increase in the use of parameter estimation rather than hypothesis testing and there was evidence that methodological rigour improved.Rehabilitation trialists are answering important questions about their interventions. Outcomes need to be more patient-centred and a measurement framework needs to be explicit. More advanced statistical methods are needed as interventions are complex. Suggestions for moving forward over the next decades are given.
Keywords: Controlled clinical trial; outcome assessment (healthcare); rehabilitation interventions.
© The Author(s) 2016.
Conflict of interest statement
Figures
Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Interventions to promote patient utilisation of cardiac rehabilitation.Cochrane Database Syst Rev. 2019 Feb 1;2(2):CD007131. doi: 10.1002/14651858.CD007131.pub4. Cochrane Database Syst Rev. 2019. PMID: 30706942 Free PMC article.
-
Evaluating the Landscape of Clinical Research in Neurosurgery.Neurosurgery. 2019 Sep 1;85(3):E485-E493. doi: 10.1093/neuros/nyz020. Neurosurgery. 2019. PMID: 30809663 Review.
-
Quality and reporting of trial design in scientific papers in Anaesthesia over 25 years.Anaesthesia. 2009 Jan;64(1):60-4. doi: 10.1111/j.1365-2044.2008.05712.x. Epub 2008 Nov 22. Anaesthesia. 2009. PMID: 19032695
-
The quality and utility of research in ectopic pregnancy in the last three decades: An analysis of the published literature.Eur J Obstet Gynecol Reprod Biol. 2020 Feb;245:134-142. doi: 10.1016/j.ejogrb.2019.12.022. Epub 2019 Dec 26. Eur J Obstet Gynecol Reprod Biol. 2020. PMID: 31901601 Review.
Cited by
-
Where have all the pilot studies gone? A follow-up on 30 years of pilot studies in Clinical Rehabilitation.Clin Rehabil. 2017 Sep;31(9):1238-1248. doi: 10.1177/0269215517692129. Epub 2017 Feb 1. Clin Rehabil. 2017. PMID: 28786333 Free PMC article.
-
Influence of humanistic care based on Carolina care model for ovarian cancer patients on postoperative recovery and quality of life.Am J Transl Res. 2021 Apr 15;13(4):3390-3399. eCollection 2021. Am J Transl Res. 2021. PMID: 34017514 Free PMC article.
-
Rationale for Intervention and Dose Is Lacking in Stroke Recovery Trials: A Systematic Review.Stroke Res Treat. 2018 Oct 30;2018:8087372. doi: 10.1155/2018/8087372. eCollection 2018. Stroke Res Treat. 2018. PMID: 30515288 Free PMC article. Review.
-
Dose Articulation in Preclinical and Clinical Stroke Recovery: Refining a Discovery Research Pipeline and Presenting a Scoping Review Protocol.Front Neurol. 2019 Nov 6;10:1148. doi: 10.3389/fneur.2019.01148. eCollection 2019. Front Neurol. 2019. PMID: 31781018 Free PMC article.
-
The Families First Program to Prevent Child Abuse: Results of a Cluster Randomized Controlled Trial in West Java, Indonesia.Prev Sci. 2022 Nov;23(8):1457-1469. doi: 10.1007/s11121-022-01433-w. Epub 2022 Sep 13. Prev Sci. 2022. PMID: 36098893 Free PMC article. Clinical Trial.
References
-
- Kocak FU, Unver B, Karatosun V. Level of evidence in four selected rehabilitation journals. Arch Phys Med Rehabil 2011; 92(2): 299–303. - PubMed
-
- Mayo NE. Randomized trials and other parallel comparisons of treatment. In: Bailar JC, Hoaglin DC. (eds) Medical uses of statistics. Hoboken, New Jersey: A John Wiley & Sons, Inc & The New England Journal of Medicine; 2009: 51–89.
-
- Mosteller F, Gilbert JP, McPeek B. Controversies in design and analysis of clinical trials. In: Shapiro SH, Louis TA. (eds) Clinical trials: Issues and approaches. New York: Marcel Dekker, 1983.
-
- Sherrington C, Herbert RD, Maher CG, Moseley AM. PEDro. A database of randomized trials and systematic reviews in physiotherapy. Man Ther 2000; 5(4): 223–226. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical