Evaluation of Alternative In Vivo Drug Screening Methodology: A Single Mouse Analysis
- PMID: 27496711
- PMCID: PMC5050128
- DOI: 10.1158/0008-5472.CAN-16-0122
Evaluation of Alternative In Vivo Drug Screening Methodology: A Single Mouse Analysis
Abstract
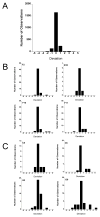
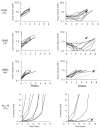
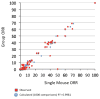
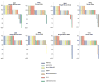
Traditional approaches to evaluating antitumor agents using human tumor xenograft models have generally used cohorts of 8 to 10 mice against a limited panel of tumor models. An alternative approach is to use fewer animals per tumor line, allowing a greater number of models that capture greater molecular/genetic heterogeneity of the cancer type. We retrospectively analyzed 67 agents evaluated by the Pediatric Preclinical Testing Program to determine whether a single mouse, chosen randomly from each group of a study, predicted the median response for groups of mice using 83 xenograft models. The individual tumor response from a randomly chosen mouse was compared with the group median response using established response criteria. A total of 2,134 comparisons were made. The single tumor response accurately predicted the group median response in 1,604 comparisons (75.16%). The mean tumor response correct prediction rate for 1,000 single mouse random samples was 78.09%. Models had a range for correct prediction (60%-87.5%). Allowing for misprediction of ± one response category, the overall mean correct single mouse prediction rate was 95.28%, and predicted overall objective response rates for group data in 66 of 67 drug studies. For molecularly targeted agents, occasional exceptional responder models were identified and the activity of that agent confirmed in additional models with the same genotype. Assuming that large treatment effects are targeted, this alternate experimental design has similar predictive value as traditional approaches, allowing for far greater numbers of models to be used that more fully encompass the heterogeneity of disease types. Cancer Res; 76(19); 5798-809. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest. The authors have no conflicts to report.
Figures
References
-
- Goldin A, Venditti JM. Progress report on the screening program at the Division of Cancer Treatment, National Cancer Institute. Cancer treatment reviews. 1980;7(4):167–76. - PubMed
-
- Goldin A, Venditti JM. The new NCI screen and its implications for clinical evaluation. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 1980;70:5–20. - PubMed
-
- Goldin A, Venditti JM. A prospective screening program: current screening and its status. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 1981;76:176–91. - PubMed
-
- Sausville EA, Burger AM. Contributions of human tumor xenografts to anticancer drug development. Cancer research. 2006;66(7):3351–4. discussion 54. - PubMed
-
- Gould SE, Junttila MR, de Sauvage FJ. Translational value of mouse models in oncology drug development. Nature medicine. 2015;21(5):431–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

