Bioenergetic Insufficiencies Due to Metabolic Alterations Regulated by the Inhibitory Receptor PD-1 Are an Early Driver of CD8(+) T Cell Exhaustion
- PMID: 27496729
- PMCID: PMC4988919
- DOI: 10.1016/j.immuni.2016.07.008
Bioenergetic Insufficiencies Due to Metabolic Alterations Regulated by the Inhibitory Receptor PD-1 Are an Early Driver of CD8(+) T Cell Exhaustion
Abstract
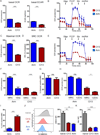
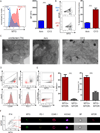
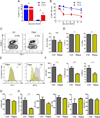
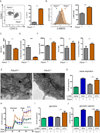
Dynamic reprogramming of metabolism is essential for T cell effector function and memory formation. However, the regulation of metabolism in exhausted CD8(+) T (Tex) cells is poorly understood. We found that during the first week of chronic lymphocytic choriomeningitis virus (LCMV) infection, before severe dysfunction develops, virus-specific CD8(+) T cells were already unable to match the bioenergetics of effector T cells generated during acute infection. Suppression of T cell bioenergetics involved restricted glucose uptake and use, despite persisting mechanistic target of rapamycin (mTOR) signaling and upregulation of many anabolic pathways. PD-1 regulated early glycolytic and mitochondrial alterations and repressed transcriptional coactivator PGC-1α. Improving bioenergetics by overexpression of PGC-1α enhanced function in developing Tex cells. Therapeutic reinvigoration by anti-PD-L1 reprogrammed metabolism in a subset of Tex cells. These data highlight a key metabolic control event early in exhaustion and suggest that manipulating glycolytic and mitochondrial metabolism might enhance checkpoint blockade outcomes.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Feeling Worn Out? PGC1α to the Rescue for Dysfunctional Mitochondria in T Cell Exhaustion.Immunity. 2016 Aug 16;45(2):233-5. doi: 10.1016/j.immuni.2016.07.024. Immunity. 2016. PMID: 27533009
-
T Cell responses: Defective mitochondria disrupt CD8(+) T cells.Nat Rev Immunol. 2016 Aug 25;16(9):534-5. doi: 10.1038/nri.2016.98. Nat Rev Immunol. 2016. PMID: 27558410 No abstract available.
References
-
- Austin S, St-Pierre J. PGC1alpha and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci. 2012;125:4963–4971. - PubMed
-
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

