LARGE2-dependent glycosylation confers laminin-binding ability on proteoglycans
- PMID: 27496765
- PMCID: PMC5137251
- DOI: 10.1093/glycob/cww075
LARGE2-dependent glycosylation confers laminin-binding ability on proteoglycans
Abstract
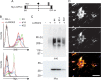
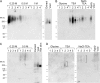
Both LARGE1 (formerly LARGE) and its paralog LARGE2 are bifunctional glycosyltransferases with xylosy- and glucuronyltransferase activities, and are capable of synthesizing polymers composed of a repeating disaccharide [-3Xylα1,3GlcAβ1-]. Post-translational modification of the O-mannosyl glycan of α-dystroglycan (α-DG) with the polysaccharide is essential for it to act as a receptor for ligands in the extracellular matrix (ECM), and both LARGE paralogs contribute to the modification in vivo. LARGE1 and LARGE2 have different tissue distribution profiles and enzymatic properties; however, the functional difference of the homologs remains to be determined, and α-DG is the only known substrate for the modification by LARGE1 or LARGE2. Here we show that LARGE2 can modify proteoglycans (PGs) with the laminin-binding glycan. We found that overexpression of LARGE2, but not LARGE1, mediates the functional modification on the surface of DG-/-, Pomt1-/- and Fktn-/- embryonic stem cells. We identified a heparan sulfate-PG glypican-4 as a substrate for the LARGE2-dependent modification by affinity purification and subsequent mass spectrometric analysis. Furthermore, we showed that LARGE2 could modify several additional PGs with the laminin-binding glycan, most likely within the glycosaminoglycan (GAG)-protein linkage region. Our results indicate that LARGE2 can modify PGs with the GAG-like polysaccharide composed of xylose and glucuronic acid to confer laminin binding. Thus, LARGE2 may play a differential role in stabilizing the basement membrane and modifying its functions by augmenting the interactions between laminin globular domain-containing ECM proteins and PGs.
Keywords: dystroglycan, glycosaminoglycan, laminin binding, LARGE2, proteoglycan.
© The Author 2016. Published by Oxford University Press.
Figures




References
-
- Ashikov A, Buettner FF, Tiemann B, Gerardy-Schahn R, Bakker H. 2013. LARGE2 generates the same xylose- and glucuronic acid-containing glycan structures as LARGE. Glycobiology. 23:303–309. - PubMed
-
- Baeuerle PA, Huttner WB. 1986. Chlorate—A potent inhibitor of protein sulfation in intact cells. Biochem Biophys Res Commun. 141:870–877. - PubMed
-
- Barresi R, Campbell KP. 2006. Dystroglycan: From biosynthesis to pathogenesis of human disease. J. Cell Sci.. 119:199–207. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

