Extending CRISPR-Cas9 Technology from Genome Editing to Transcriptional Engineering in the Genus Clostridium
- PMID: 27496775
- PMCID: PMC5068152
- DOI: 10.1128/AEM.02128-16
Extending CRISPR-Cas9 Technology from Genome Editing to Transcriptional Engineering in the Genus Clostridium
Abstract
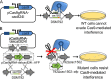
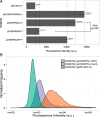
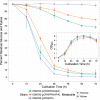
The discovery and exploitation of the prokaryotic adaptive immunity system based on clustered regularly interspaced short palindromic repeats (CRISPRs) and CRISPR-associated (Cas) proteins have revolutionized genetic engineering. CRISPR-Cas tools have enabled extensive genome editing as well as efficient modulation of the transcriptional program in a multitude of organisms. Progress in the development of genetic engineering tools for the genus Clostridium has lagged behind that of many other prokaryotes, presenting the CRISPR-Cas technology an opportunity to resolve a long-existing issue. Here, we applied the Streptococcus pyogenes type II CRISPR-Cas9 (SpCRISPR-Cas9) system for genome editing in Clostridium acetobutylicum DSM792. We further explored the utility of the SpCRISPR-Cas9 machinery for gene-specific transcriptional repression. For proof-of-concept demonstration, a plasmid-encoded fluorescent protein gene was used for transcriptional repression in C. acetobutylicum Subsequently, we targeted the carbon catabolite repression (CCR) system of C. acetobutylicum through transcriptional repression of the hprK gene encoding HPr kinase/phosphorylase, leading to the coutilization of glucose and xylose, which are two abundant carbon sources from lignocellulosic feedstocks. Similar approaches based on SpCRISPR-Cas9 for genome editing and transcriptional repression were also demonstrated in Clostridium pasteurianum ATCC 6013. As such, this work lays a foundation for the derivation of clostridial strains for industrial purposes.
Importance: After recognizing the industrial potential of Clostridium for decades, methods for the genetic manipulation of these anaerobic bacteria are still underdeveloped. This study reports the implementation of CRISPR-Cas technology for genome editing and transcriptional regulation in Clostridium acetobutylicum, which is arguably the most common industrial clostridial strain. The developed genetic tools enable simpler, more reliable, and more extensive derivation of C. acetobutylicum mutant strains for industrial purposes. Similar approaches were also demonstrated in Clostridium pasteurianum, another clostridial strain that is capable of utilizing glycerol as the carbon source for butanol fermentation, and therefore can be arguably applied in other clostridial strains.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

