Incisional negative pressure wound therapy dressings (iNPWTd) in routine primary hip and knee arthroplasties: A randomised controlled trial
- PMID: 27496913
- PMCID: PMC5013893
- DOI: 10.1302/2046-3758.58.BJR-2016-0022.R1
Incisional negative pressure wound therapy dressings (iNPWTd) in routine primary hip and knee arthroplasties: A randomised controlled trial
Abstract
Objectives: Wound complications are reported in up to 10% hip and knee arthroplasties and there is a proven association between wound complications and deep prosthetic infections. In this randomised controlled trial (RCT) we explore the potential benefits of a portable, single use, incisional negative pressure wound therapy dressing (iNPWTd) on wound exudate, length of stay (LOS), wound complications, dressing changes and cost-effectiveness following total hip and knee arthroplasties.
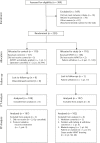
Methods: A total of 220 patients undergoing elective primary total hip and knee arthroplasties were recruited into in a non-blinded RCT. For the final analysis there were 102 patients in the study group and 107 in the control group.
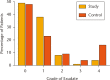
Results: An improvement was seen in the study (iNPWTd) group compared to control in all areas. Peak post-surgical wound exudate was significantly reduced (p = 0.007). Overall LOS reduction (0.9 days, 95% confidence interval (CI) -0.2 to 2.5) was not significant (p = 0.07) but there was a significant reduction in patients with extreme values of LOS in the iNPWTd group (Moses test, p = 0.003). There was a significantly reduced number of dressing changes (mean difference 1.7, 95% CI 0.8 to 2.5, p = 0.002), and a trend to a significant four-fold reduction in reported post-operative surgical wound complications (8.4% control; 2.0% iNPWTd, p = 0.06).
Conclusions: Based on the results of this RCT incisional negative pressure wound therapy dressings have a beneficial role in patients undergoing primary hip and knee arthroplasty to achieve predictable length of stay, especially to eliminate excessive hospital stay, and minimise wound complications.Cite this article: S. L. Karlakki, A. K. Hamad, C. Whittall, N. M. Graham, R. D. Banerjee, J. H. Kuiper. Incisional negative pressure wound therapy dressings (iNPWTd) in routine primary hip and knee arthroplasties: A randomised controlled trial. Bone Joint Res 2016;5:328-337. DOI: 10.1302/2046-3758.58.BJR-2016-0022.R1.
Keywords: Incisional wound; NPWT; THA; TKA; Wound complications; iNPWTd.
© 2016 Karlakki et al.
Conflict of interest statement
ICMJE conflict of interest: None declared.
Figures
References
-
- Norman-Taylor FH, Palmer CR, Villar RN. Quality-of-life improvement compared after hip and knee replacement. J Bone Joint Surg [Br] 1996;78-B:74-77. - PubMed
-
- Patel VP, Walsh M, Sehgal B, et al. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg [Am] 2007;89-A:33-38. - PubMed
-
- Weiss AP, Krackow KA. Persistent wound drainage after primary total knee arthroplasty. J Arthroplasty 1993;8:285-289. - PubMed
-
- Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res 2002;20:506-515. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical