Conformational Sub-states and Populations in Enzyme Catalysis
- PMID: 27497171
- PMCID: PMC4977995
- DOI: 10.1016/bs.mie.2016.05.023
Conformational Sub-states and Populations in Enzyme Catalysis
Abstract
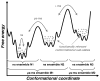
Enzyme function involves substrate and cofactor binding, precise positioning of reactants in the active site, chemical turnover, and release of products. In addition to formation of crucial structural interactions between enzyme and substrate(s), coordinated motions within the enzyme-substrate complex allow reaction to proceed at a much faster rate, compared to the reaction in solution and in the absence of enzyme. An increasing number of enzyme systems show the presence of conserved protein motions that are important for function. A wide variety of motions are naturally sampled (over femtosecond to millisecond time-scales) as the enzyme complex moves along the energetic landscape, driven by temperature and dynamical events from the surrounding environment. Areas of low energy along the landscape form conformational sub-states, which show higher conformational populations than surrounding areas. A small number of these protein conformational sub-states contain functionally important structural and dynamical features, which assist the enzyme mechanism along the catalytic cycle. Identification and characterization of these higher-energy (also called excited) sub-states and the associated populations are challenging, as these sub-states are very short-lived and therefore rarely populated. Specialized techniques based on computer simulations, theoretical modeling, and nuclear magnetic resonance have been developed for quantitative characterization of these sub-states and populations. This chapter discusses these techniques and provides examples of their applications to enzyme systems.
Keywords: Computational modeling; Conformational dynamics; Conformational population; Conformational substates; Enzyme dynamics; Nuclear magnetic resonance; Protein relaxation.
© 2016 Elsevier Inc. All rights reserved.
Figures





References
-
- Agarwal PK. Cis/trans isomerization in HIV-1 capsid protein catalyzed by cyclophilin A: Insights from computational and theoretical studies. Proteins-Structure Function and Bioinformatics. 2004;56(3):449–463. - PubMed
-
- Agarwal PK. Role of protein dynamics in reaction rate enhancement by enzymes. Journal of the American Chemical Society. 2005;127(43):15248–15256. - PubMed
-
- Agarwal PK, Geist A, Gorin A. Protein dynamics and enzymatic catalysis: Investigating the peptidyl-prolyl cis-trans isomerization activity of cyclophilin A. Biochemistry. 2004;43(33):10605–10618. - PubMed
-
- Benkovic SJ. Catalytic Antibodies. Annual Review of Biochemistry. 1992;61:29–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

