Hyperphosphorylated tau in patients with refractory epilepsy correlates with cognitive decline: a study of temporal lobe resections
- PMID: 27497924
- PMCID: PMC5926008
- DOI: 10.1093/brain/aww187
Hyperphosphorylated tau in patients with refractory epilepsy correlates with cognitive decline: a study of temporal lobe resections
Abstract
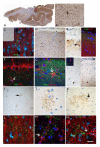
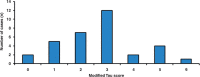
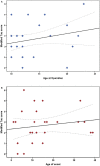
SEE BERNASCONI DOI101093/AWW202 FOR A SCIENTIFIC COMMENTARY ON THIS ARTICLE: Temporal lobe epilepsy, the most prevalent form of chronic focal epilepsy, is associated with a high prevalence of cognitive impairment but the responsible underlying pathological mechanisms are unknown. Tau, the microtubule-associated protein, is a hallmark of several neurodegenerative diseases including Alzheimer's disease and chronic traumatic encephalopathy. We hypothesized that hyperphosphorylated tau pathology is associated with cognitive decline in temporal lobe epilepsy and explored this through clinico-pathological study. We first performed pathological examination on tissue from 33 patients who had undergone temporal lobe resection between ages 50 and 65 years to treat drug-refractory temporal lobe epilepsy. We identified hyperphosphorylated tau protein using AT8 immunohistochemistry and compared this distribution to Braak patterns of Alzheimer's disease and patterns of chronic traumatic encephalopathy. We quantified tau pathology using a modified tau score created specifically for analysis of temporal lobectomy tissue and the Braak staging, which was limited without extra-temporal brain areas available. Next, we correlated tau pathology with pre- and postoperative cognitive test scores and clinical risk factors including age at time of surgery, duration of epilepsy, history of secondary generalized seizures, history of head injury, handedness and side of surgery. Thirty-one of 33 cases (94%) showed hyperphosphorylated tau pathology in the form of neuropil threads and neurofibrillary tangles and pre-tangles. Braak stage analysis showed 12% of our epilepsy cohort had a Braak staging III-IV compared to an age-matched non-epilepsy control group from the literature (8%). We identified a mixture of tau pathology patterns characteristic of Alzheimer's disease and chronic traumatic encephalopathy. We also found unusual patterns of subpial tau deposition, sparing of the hippocampus and co-localization with mossy fibre sprouting, a feature of temporal lobe epilepsy. We demonstrated that the more extensive the tau pathology, the greater the decline in verbal learning (Spearman correlation, r = -0.63), recall (r = -0.44) and graded naming test scores (r = -0.50) over 1-year post-temporal lobe resection (P < 0.05). This relationship with tau burden was also present when examining decline in verbal learning from 3 months to 1 year post-resection (r = -0.54). We found an association between modified tau score and history of secondary generalized seizures (likelihood-ratio χ(2), P < 0.05) however there was no clear relationship between tau pathology and other clinical risk factors assessed. Our findings suggest an epilepsy-related tauopathy in temporal lobe epilepsy, which contributes to accelerated cognitive decline and has diagnostic and treatment implications.
Keywords: Alzheimer’s disease; dementia; neurofibrillary tangles; tau; temporal lobe epilepsy.
© The Author (2016). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



Comment in
-
Is epilepsy a curable neurodegenerative disease?Brain. 2016 Sep;139(Pt 9):2336-7. doi: 10.1093/brain/aww202. Brain. 2016. PMID: 27559105 No abstract available.
-
Do Patients With Temporal Lobe Epilepsy and Cognitive Decline Have Alzheimer's Disease or Chronic Traumatic Encephalopathy (CTE)?Epilepsy Curr. 2017 Mar-Apr;17(2):96-98. doi: 10.5698/1535-7511.17.2.96. Epilepsy Curr. 2017. PMID: 28490998 Free PMC article. No abstract available.
-
Seizures in Alzheimer's disease: is there more beneath the surface?J Neurol. 2018 Jan;265(1):226-228. doi: 10.1007/s00415-017-8694-6. J Neurol. 2018. PMID: 29204960 Free PMC article. No abstract available.
References
-
- Amatniek JC, Hauser WA, DelCastillo-Castaneda C, Jacobs DM, Marder K, Bell K, et al. . Incidence and predictors of seizures in patients with Alzheimer’s disease [Internet]. Epilepsia 2006; 47: 867–72. - PubMed
-
- Black LC, Schefft BK, Howe SR, Szaflarski JP, Yeh H, Privitera MD. The effect of seizures on working memory and executive functioning performance [Internet]. Epilepsy Behav 2010; 17: 412–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

