Active surveillance in metastatic renal-cell carcinoma: a prospective, phase 2 trial
- PMID: 27498080
- PMCID: PMC9199453
- DOI: 10.1016/S1470-2045(16)30196-6
Active surveillance in metastatic renal-cell carcinoma: a prospective, phase 2 trial
Abstract
Background: A subset of patients with metastatic renal-cell carcinoma show indolent growth of metastases. Because of the toxicity and non-curative nature of systemic therapy, some of these patients could benefit from initial active surveillance. We aimed to characterise the time to initiation of systemic therapy in patients with metastatic renal-cell carcinoma under active surveillance.
Methods: In this prospective phase 2 trial, we enrolled patients with treatment-naive, asymptomatic, metastatic renal-cell carcinoma from five hospitals in the USA, Spain, and the UK. Patients were radiographically assessed at baseline, every 3 months for year 1, every 4 months for year 2, then every 6 months thereafter. Patients continued on observation until initiation of systemic therapy for metastatic renal-cell carcinoma; a decision that was made at the discretion of the treating physician and patient. The primary endpoint of the study was time to initiation of systemic therapy in the per-protocol population. The follow-up of patients is ongoing.
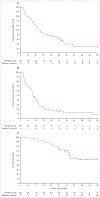
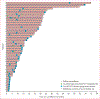
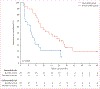
Findings: Between Aug 21, 2008, and June 7, 2013, we enrolled 52 patients. Median follow-up of patients in the study was 38·1 months (IQR 29·4-48·9). In the 48 patients included in analysis, median time on surveillance from registration on study until initiation of systemic therapy was 14·9 months (95% CI 10·6-25·0). Multivariate analysis showed that higher numbers of International Metastatic Database Consortium (IMDC) adverse risk factors (p=0·0403) and higher numbers of metastatic disease sites (p=0·0414) were associated with a shorter surveillance period. 22 (46%) patients died during the study period, all from metastatic renal-cell carcinoma.
Interpretation: A subset of patients with metastatic renal-cell carcinoma can safely undergo surveillance before starting systemic therapy. Additional investigation is required to further define the benefits and risks of this approach.
Funding: None.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Delayed systemic treatment in metastatic renal-cell carcinoma.Lancet Oncol. 2016 Sep;17(9):1187-9. doi: 10.1016/S1470-2045(16)30247-9. Epub 2016 Aug 3. Lancet Oncol. 2016. PMID: 27498081 No abstract available.
-
Re: Brian I. Rini, Tanya B. Dorff, Paul Elson, et al. Active Surveillance in Metastatic Renal-cell Carcinoma: A Prospective, Phase 2 Trial. Lancet Oncol Lancet 2016;17:1317-24: Active Surveillance in Metastatic Renal Cell Carcinoma: Option or Exception?Eur Urol. 2017 May;71(5):e139-e140. doi: 10.1016/j.eururo.2016.09.034. Epub 2016 Sep 29. Eur Urol. 2017. PMID: 27692706 No abstract available.
References
-
- Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013; 369: 722–31. - PubMed
-
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastati c renal-cell carcinoma. N Engl J Med 2007; 356: 115–24. - PubMed
-
- Rini BI, Escudier B, Tomczak P, et al. Comparative eff ectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011; 378: 1931–39. - PubMed
-
- Dabestani S, Marconi L, Hofmann F, et al. Local treatments for metastases of renal c ell carcinoma: a systematic review. Lancet Oncol 2014; 15: e549–61. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

