Known unknowns of cardiolipin signaling: The best is yet to come
- PMID: 27498292
- PMCID: PMC5323096
- DOI: 10.1016/j.bbalip.2016.08.001
Known unknowns of cardiolipin signaling: The best is yet to come
Abstract
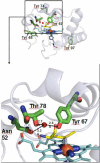
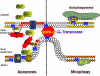
Since its discovery 75years ago, a wealth of knowledge has accumulated on the role of cardiolipin, the hallmark phospholipid of mitochondria, in bioenergetics and particularly on the structural organization of the inner mitochondrial membrane. A surge of interest in this anionic doubly-charged tetra-acylated lipid found in both prokaryotes and mitochondria has emerged based on its newly discovered signaling functions. Cardiolipin displays organ, tissue, cellular and transmembrane distribution asymmetries. A collapse of the membrane asymmetry represents a pro-mitophageal mechanism whereby externalized cardiolipin acts as an "eat-me" signal. Oxidation of cardiolipin's polyunsaturated acyl chains - catalyzed by cardiolipin complexes with cytochrome c. - is a pro-apoptotic signal. The messaging functions of myriads of cardiolipin species and their oxidation products are now being recognized as important intracellular and extracellular signals for innate and adaptive immune systems. This newly developing field of research exploring cardiolipin signaling is the main subject of this review. This article is part of a Special Issue entitled: Lipids of Mitochondria edited by Guenther Daum.
Keywords: Apoptosis; Cardiolipin oxidation; Cardiolipin signaling; Innate and adaptive immunity; Mitophagy; Peroxidase.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures




References
-
- Lane N, Martin W. The energetics of genome complexity. Nature. 2010;467:929–934. - PubMed
-
- Lyons TW, Reinhard CT, Planavsky NJ. The rise of oxygen in Earth's early ocean and atmosphere. Nature. 2014;506:307–315. - PubMed
-
- Westbroek P. Life as a geological force — dynamics of the earth. W. W. Norton; New York, London: 1991.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

