Human Cytomegalovirus nuclear egress and secondary envelopment are negatively affected in the absence of cellular p53
- PMID: 27498410
- PMCID: PMC5026618
- DOI: 10.1016/j.virol.2016.07.021
Human Cytomegalovirus nuclear egress and secondary envelopment are negatively affected in the absence of cellular p53
Abstract
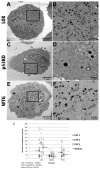
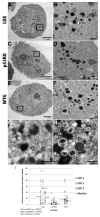
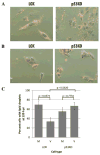
Human Cytomegalovirus (HCMV) infection is compromised in cells lacking p53, a transcription factor that mediates cellular stress responses. In this study we have investigated compromised functional virion production in cells with p53 knocked out (p53KOs). Infectious center assays found most p53KOs released functional virions. Analysis of electron micrographs revealed modestly decreased capsid production in infected p53KOs compared to wt. Substantially fewer p53KOs displayed HCMV-induced infoldings of the inner nuclear membrane (IINMs). In p53KOs, fewer capsids were found in IINMs and in the cytoplasm. The deficit in virus-induced membrane remodeling within the nucleus of p53KOs was mirrored in the cytoplasm, with a disproportionately smaller number of capsids re-enveloped. Reintroduction of p53 substantially recovered these deficits. Overall, the absence of p53 contributed to inhibition of the formation and function of IINMs and re-envelopment of the reduced number of capsids able to reach the cytoplasm.
Keywords: Capsids; Human Cytomegalovirus; Infoldings of the inner nuclear membrane (IINM); Nuclear egress; Secondary envelopment; Transmission electron microscopy (TEM); p53.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Human Cytomegalovirus Egress: Overcoming Barriers and Co-Opting Cellular Functions.Viruses. 2021 Dec 22;14(1):15. doi: 10.3390/v14010015. Viruses. 2021. PMID: 35062219 Free PMC article. Review.
-
The absence of p53 during Human Cytomegalovirus infection leads to decreased UL53 expression, disrupting UL50 localization to the inner nuclear membrane, and thereby inhibiting capsid nuclear egress.Virology. 2016 Oct;497:262-278. doi: 10.1016/j.virol.2016.07.020. Epub 2016 Aug 4. Virology. 2016. PMID: 27498409 Free PMC article.
-
A Role for Myosin Va in Human Cytomegalovirus Nuclear Egress.J Virol. 2018 Feb 26;92(6):e01849-17. doi: 10.1128/JVI.01849-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29298889 Free PMC article.
-
WDR5 Facilitates Human Cytomegalovirus Replication by Promoting Capsid Nuclear Egress.J Virol. 2018 Apr 13;92(9):e00207-18. doi: 10.1128/JVI.00207-18. Print 2018 May 1. J Virol. 2018. PMID: 29437978 Free PMC article.
-
Herpesvirus Nuclear Egress across the Outer Nuclear Membrane.Viruses. 2021 Nov 24;13(12):2356. doi: 10.3390/v13122356. Viruses. 2021. PMID: 34960625 Free PMC article. Review.
Cited by
-
Emerging Mechanisms of G1/S Cell Cycle Control by Human and Mouse Cytomegaloviruses.mBio. 2021 Dec 21;12(6):e0293421. doi: 10.1128/mBio.02934-21. Epub 2021 Dec 14. mBio. 2021. PMID: 34903047 Free PMC article. Review.
-
Human Cytomegalovirus Egress: Overcoming Barriers and Co-Opting Cellular Functions.Viruses. 2021 Dec 22;14(1):15. doi: 10.3390/v14010015. Viruses. 2021. PMID: 35062219 Free PMC article. Review.
-
Human herpesvirus 6A nuclear matrix protein U37 interacts with heat shock transcription factor 1 and activates the heat shock response.J Virol. 2023 Sep 28;97(9):e0071823. doi: 10.1128/jvi.00718-23. Epub 2023 Sep 6. J Virol. 2023. PMID: 37671864 Free PMC article.
-
Novel modulators of p53-signaling encoded by unknown genes of emerging viruses.PLoS Pathog. 2021 Jan 7;17(1):e1009033. doi: 10.1371/journal.ppat.1009033. eCollection 2021 Jan. PLoS Pathog. 2021. PMID: 33411764 Free PMC article.
-
Murine Cytomegalovirus M25 Proteins Sequester the Tumor Suppressor Protein p53 in Nuclear Accumulations.J Virol. 2020 Sep 29;94(20):e00574-20. doi: 10.1128/JVI.00574-20. Print 2020 Sep 29. J Virol. 2020. PMID: 32727874 Free PMC article.
References
-
- Adler B, Sinzger C. Cytomegalovirus Interstrain Variance in Cell Type Tropism. In: Reddehase MJ, Lemmermann NAW, editors. Cytomegaloviruses: From Molecular Pathogenesis to Intervention. Caister Academic Press; Norfolk, UK: 2013. pp. 297–321.
-
- Azzeh M, Honigman A, Taraboulos A, Rouvinski A, Wolf DG. Structural changes in human cytomegalovirus cytoplasmic assembly sites in the absence of UL97 kinase activity. Virology. 2006;354:69–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

