The structural basis of the dominant negative phenotype of the Gαi1β1γ2 G203A/A326S heterotrimer
- PMID: 27498775
- PMCID: PMC5022103
- DOI: 10.1038/aps.2016.69
The structural basis of the dominant negative phenotype of the Gαi1β1γ2 G203A/A326S heterotrimer
Abstract
Aim: Dominant negative mutant G proteins have provided critical insight into the mechanisms of G protein-coupled receptor (GPCR) signaling, but the mechanisms underlying the dominant negative characteristics are not completely understood. The aim of this study was to determine the structure of the dominant negative Gαi1β1γ2 G203A/A326S complex (Gi-DN) and to reveal the structural basis of the mutation-induced phenotype of Gαi1β1γ2.
Methods: The three subunits of the Gi-DN complex were co-expressed with a baculovirus expression system. The Gi-DN heterotrimer was purified, and the structure of its complex with GDP was determined through X-ray crystallography.
Results: The Gi-DN heterotrimer structure revealed a dual mechanism underlying the dominant negative characteristics. The mutations weakened the hydrogen bonding network between GDP/GTP and the binding pocket residues, and increased the interactions in the Gα-Gβγ interface. Concomitantly, the Gi-DN heterotrimer adopted a conformation, in which the C-terminus of Gαi and the N-termini of both the Gβ and Gγ subunits were more similar to the GPCR-bound state compared with the wild type complex. From these structural observations, two additional mutations (T48F and D272F) were designed that completely abolish the GDP binding of the Gi-DN heterotrimer.
Conclusion: Overall, the results suggest that the mutations impede guanine nucleotide binding and Gα-Gβγ protein dissociation and favor the formation of the G protein/GPCR complex, thus blocking signal propagation. In addition, the structure provides a rationale for the design of other mutations that cause dominant negative effects in the G protein, as exemplified by the T48F and D272F mutations.
Figures


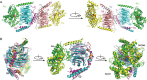





Similar articles
-
Evidence for a second, high affinity Gbetagamma binding site on Galphai1(GDP) subunits.J Biol Chem. 2009 Jun 19;284(25):16906-16913. doi: 10.1074/jbc.M109.006585. Epub 2009 Apr 15. J Biol Chem. 2009. PMID: 19369247 Free PMC article.
-
Dissociated GαGTP and Gβγ protein subunits are the major activated form of heterotrimeric Gi/o proteins.J Biol Chem. 2014 Jan 17;289(3):1271-81. doi: 10.1074/jbc.M113.493643. Epub 2013 Dec 4. J Biol Chem. 2014. PMID: 24307173 Free PMC article.
-
The A326S mutant of Gialpha1 as an approximation of the receptor-bound state.J Biol Chem. 1998 Aug 21;273(34):21752-8. doi: 10.1074/jbc.273.34.21752. J Biol Chem. 1998. PMID: 9705312
-
Mechanisms of dominant negative G-protein alpha subunits.J Neurosci Res. 2007 Dec;85(16):3505-14. doi: 10.1002/jnr.21414. J Neurosci Res. 2007. PMID: 17639598 Review.
-
Chaperone-mediated assembly of G protein complexes.Subcell Biochem. 2012;63:131-53. doi: 10.1007/978-94-007-4765-4_8. Subcell Biochem. 2012. PMID: 23161137 Review.
Cited by
-
Structural insights into the human D1 and D2 dopamine receptor signaling complexes.Cell. 2021 Feb 18;184(4):931-942.e18. doi: 10.1016/j.cell.2021.01.027. Epub 2021 Feb 10. Cell. 2021. PMID: 33571431 Free PMC article.
-
Structural basis of the ligand binding and signaling mechanism of melatonin receptors.Nat Commun. 2022 Jan 24;13(1):454. doi: 10.1038/s41467-022-28111-3. Nat Commun. 2022. PMID: 35075127 Free PMC article.
-
Molecular basis for kinin selectivity and activation of the human bradykinin receptors.Nat Struct Mol Biol. 2021 Sep;28(9):755-761. doi: 10.1038/s41594-021-00645-y. Epub 2021 Sep 9. Nat Struct Mol Biol. 2021. PMID: 34518695
-
Structural insights into ligand recognition and activation of the medium-chain fatty acid-sensing receptor GPR84.Nat Commun. 2023 Jun 6;14(1):3271. doi: 10.1038/s41467-023-38985-6. Nat Commun. 2023. PMID: 37277332 Free PMC article.
-
Mechanistic Insights into Side Effects of Troglitazone and Rosiglitazone Using a Novel Inverse Molecular Docking Protocol.Pharmaceutics. 2021 Feb 28;13(3):315. doi: 10.3390/pharmaceutics13030315. Pharmaceutics. 2021. PMID: 33670968 Free PMC article.
References
-
- Sunahara RK, Tesmer JJ, Gilman AG, Sprang SR. Crystal structure of the adenylyl cyclase activator Gsalpha. Science 1997; 278: 1943–7. - PubMed
-
- Coleman DE, Berghuis AM, Lee E, Linder ME, Gilman AG, Sprang SR. Structures of active conformations of Gi alpha 1 and the mechanism of GTP hydrolysis. Science 1994; 265: 1405–12. - PubMed
-
- Noel JP, Hamm HE, Sigler PB. The 2.2 A crystal structure of transducin-alpha complexed with GTP gamma S. Nature 1993; 366: 654–63. - PubMed
-
- Mixon MB, Lee E, Coleman DE, Berghuis AM, Gilman AG, Sprang SR. Tertiary and quaternary structural changes in Gi alpha 1 induced by GTP hydrolysis. Science 1995; 270: 954–60. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

