Deciphering Variability of PKD1 and PKD2 in an Italian Cohort of 643 Patients with Autosomal Dominant Polycystic Kidney Disease (ADPKD)
- PMID: 27499327
- PMCID: PMC4976333
- DOI: 10.1038/srep30850
Deciphering Variability of PKD1 and PKD2 in an Italian Cohort of 643 Patients with Autosomal Dominant Polycystic Kidney Disease (ADPKD)
Abstract
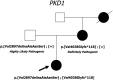
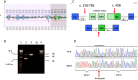
Autosomal Dominant Polycystic Kidney Disease (ADPKD) is the most common hereditary kidney disease. We analysed PKD1 and PKD2, in a large cohort of 440 unrelated Italian patients with ADPKD and 203 relatives by direct sequencing and MLPA. Molecular and detailed phenotypic data have been collected and submitted to the PKD1/PKD2 LOVD database. This is the first large retrospective study in Italian patients, describing 701 variants, 249 (35.5%) already associated with ADPKD and 452 (64.5%) novel. According to the criteria adopted, the overall detection rate was 80% (352/440). Novel variants with uncertain significance were found in 14% of patients. Among patients with pathogenic variants, in 301 (85.5%) the disease is associated with PKD1, 196 (55.7%) truncating, 81 (23%) non truncating, 24 (6.8%) IF indels, and in 51 (14.5%) with PKD2. Our results outline the high allelic heterogeneity of variants, complicated by the presence of variants of uncertain significance as well as of multiple variants in the same subject. Classification of novel variants may be particularly cumbersome having an important impact on the genetic counselling. Our study confirms the importance to improve the assessment of variant pathogenicity for ADPKD; to this point databasing of both clinical and molecular data is crucial.
Figures




References
-
- Torres V. E., Harris P. C. & Pirson Y. Autosomal dominant polycystic kidney disease. The Lancet 369, 1287–1301 (2007). - PubMed
-
- Bergmann C. & Zerres K. Early manifestations of polycystic kidney disease. The Lancet 369, 2157 (2007). - PubMed
-
- Bergmann C., Ortiz Bruchle N., Frank V., Rehder H. & Zerres K. Perinatal deaths in a family with autosomal dominant polycystic kidney disease and a PKD2 mutation. N. Engl. J. Med. 359, 318–319 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

