Tankyrase Sterile α Motif Domain Polymerization Is Required for Its Role in Wnt Signaling
- PMID: 27499439
- PMCID: PMC5109827
- DOI: 10.1016/j.str.2016.06.022
Tankyrase Sterile α Motif Domain Polymerization Is Required for Its Role in Wnt Signaling
Abstract
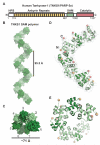
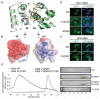
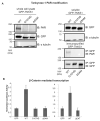
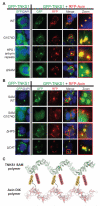
Tankyrase-1 (TNKS1/PARP-5a) is a poly(ADP-ribose) polymerase (PARP) enzyme that regulates multiple cellular processes creating a poly(ADP-ribose) posttranslational modification that can lead to target protein turnover. TNKS1 thereby controls protein levels of key components of signaling pathways, including Axin1, the limiting component of the destruction complex in canonical Wnt signaling that degrades β-catenin to prevent its coactivator function in gene expression. There are limited molecular level insights into TNKS1 regulation in cell signaling pathways. TNKS1 has a sterile α motif (SAM) domain that is known to mediate polymerization, but the functional requirement for SAM polymerization has not been assessed. We have determined the crystal structure of wild-type human TNKS1 SAM domain and used structure-based mutagenesis to disrupt polymer formation and assess the consequences on TNKS1 regulation of β-catenin-dependent transcription. Our data indicate the SAM polymer is critical for TNKS1 catalytic activity and allows TNKS1 to efficiently access cytoplasmic signaling complexes.
Keywords: Wnt/β-catenin signaling; X-ray crystallography; poly(ADP-ribose) polymerase; tankyrase.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
References
-
- Bienz M. Signalosome assembly by domains undergoing dynamic head-to-tail polymerization. Trends Biochem. Sci. 2014;39:487–495. - PubMed
-
- Chang P, Coughlin M, Mitchison TJ. Tankyrase-1 polymerization of poly(ADP-ribose) is required for spindle structure and function. Nat. Cell Biol. 2005;7:1133–1139. - PubMed
-
- Chi NW, Lodish HF. Tankyrase is a golgi-associated mitogen-activated protein kinase substrate that interacts with IRAP in GLUT4 vesicles. J. Biol. Chem. 2000;275:38437–38444. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

