The cellular and compartmental profile of mouse retinal glycolysis, tricarboxylic acid cycle, oxidative phosphorylation, and ~P transferring kinases
- PMID: 27499608
- PMCID: PMC4961465
The cellular and compartmental profile of mouse retinal glycolysis, tricarboxylic acid cycle, oxidative phosphorylation, and ~P transferring kinases
Abstract
Purpose: The homeostatic regulation of cellular ATP is achieved by the coordinated activity of ATP utilization, synthesis, and buffering. Glucose is the major substrate for ATP synthesis through glycolysis and oxidative phosphorylation (OXPHOS), whereas intermediary metabolism through the tricarboxylic acid (TCA) cycle utilizes non-glucose-derived monocarboxylates, amino acids, and alpha ketoacids to support mitochondrial ATP and GTP synthesis. Cellular ATP is buffered by specialized equilibrium-driven high-energy phosphate (~P) transferring kinases. Our goals were twofold: 1) to characterize the gene expression, protein expression, and activity of key synthesizing and regulating enzymes of energy metabolism in the whole mouse retina, retinal compartments, and/or cells and 2) to provide an integrative analysis of the results related to function.
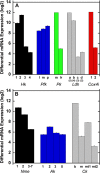
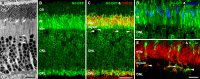
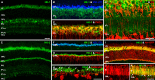
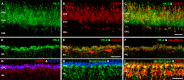
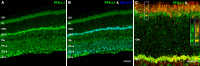
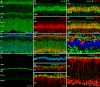
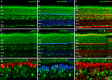
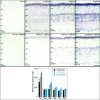
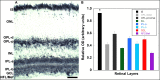
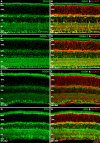
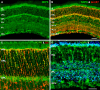
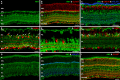
Methods: mRNA expression data of energy-related genes were extracted from our whole retinal Affymetrix microarray data. Fixed-frozen retinas from adult C57BL/6N mice were used for immunohistochemistry, laser scanning confocal microscopy, and enzymatic histochemistry. The immunoreactivity levels of well-characterized antibodies, for all major retinal cells and their compartments, were obtained using our established semiquantitative confocal and imaging techniques. Quantitative cytochrome oxidase (COX) and lactate dehydrogenase (LDH) activity was determined histochemically.
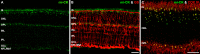
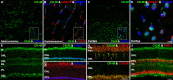
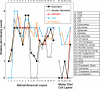
Results: The Affymetrix data revealed varied gene expression patterns of the ATP synthesizing and regulating enzymes found in the muscle, liver, and brain. Confocal studies showed differential cellular and compartmental distribution of isozymes involved in glucose, glutamate, glutamine, lactate, and creatine metabolism. The pattern and intensity of the antibodies and of the COX and LDH activity showed the high capacity of photoreceptors for aerobic glycolysis and OXPHOS. Competition assays with pyruvate revealed that LDH-5 was localized in the photoreceptor inner segments. The combined results indicate that glycolysis is regulated by the compartmental expression of hexokinase 2, pyruvate kinase M1, and pyruvate kinase M2 in photoreceptors, whereas the inner retinal neurons exhibit a lower capacity for glycolysis and aerobic glycolysis. Expression of nucleoside diphosphate kinase, mitochondria-associated adenylate kinase, and several mitochondria-associated creatine kinase isozymes was highest in the outer retina, whereas expression of cytosolic adenylate kinase and brain creatine kinase was higher in the cones, horizontal cells, and amacrine cells indicating the diversity of ATP-buffering strategies among retinal neurons. Based on the antibody intensities and the COX and LDH activity, Müller glial cells (MGCs) had the lowest capacity for glycolysis, aerobic glycolysis, and OXPHOS. However, they showed high expression of glutamate dehydrogenase, alpha-ketoglutarate dehydrogenase, succinate thiokinase, GABA transaminase, and ~P transferring kinases. This suggests that MGCs utilize TCA cycle anaplerosis and cataplerosis to generate GTP and ~P transferring kinases to produce ATP that supports MGC energy requirements.
Conclusions: Our comprehensive and integrated results reveal that the adult mouse retina expresses numerous isoforms of ATP synthesizing, regulating, and buffering genes; expresses differential cellular and compartmental levels of glycolytic, OXPHOS, TCA cycle, and ~P transferring kinase proteins; and exhibits differential layer-by-layer LDH and COX activity. New insights into cell-specific and compartmental ATP and GTP production, as well as utilization and buffering strategies and their relationship with known retinal and cellular functions, are discussed. Developing therapeutic strategies for neuroprotection and treating retinal deficits and degeneration in a cell-specific manner will require such knowledge. This work provides a platform for future research directed at identifying the molecular targets and proteins that regulate these processes.
Figures

Similar articles
-
Selective knockdown of hexokinase 2 in rods leads to age-related photoreceptor degeneration and retinal metabolic remodeling.Cell Death Dis. 2020 Oct 20;11(10):885. doi: 10.1038/s41419-020-03103-7. Cell Death Dis. 2020. PMID: 33082308 Free PMC article.
-
α-Lactalbumin-oleic acid complex kills tumor cells by inducing excess energy metabolism but inhibiting mRNA expression of the related enzymes.J Dairy Sci. 2018 Jun;101(6):4853-4863. doi: 10.3168/jds.2017-13731. Epub 2018 Mar 15. J Dairy Sci. 2018. PMID: 29550120
-
Tricarboxylic acid cycle-sustained oxidative phosphorylation in isolated myelin vesicles.Biochimie. 2013 Nov;95(11):1991-8. doi: 10.1016/j.biochi.2013.07.003. Epub 2013 Jul 12. Biochimie. 2013. PMID: 23851157
-
Mitochondria and diabetes. Genetic, biochemical, and clinical implications of the cellular energy circuit.Diabetes. 1996 Feb;45(2):113-26. doi: 10.2337/diab.45.2.113. Diabetes. 1996. PMID: 8549853 Review.
-
Reprogramming of glucose metabolism of cumulus cells and oocytes and its therapeutic significance.Reprod Sci. 2022 Mar;29(3):653-667. doi: 10.1007/s43032-021-00505-6. Epub 2021 Mar 5. Reprod Sci. 2022. PMID: 33675030 Review.
Cited by
-
The Hippo Pathway Blocks Mammalian Retinal Müller Glial Cell Reprogramming.Cell Rep. 2019 May 7;27(6):1637-1649.e6. doi: 10.1016/j.celrep.2019.04.047. Cell Rep. 2019. PMID: 31067451 Free PMC article.
-
Genetic correlation between circulating metabolites and chalazion: a two-sample Mendelian randomization study.Front Mol Biosci. 2024 Mar 21;11:1368669. doi: 10.3389/fmolb.2024.1368669. eCollection 2024. Front Mol Biosci. 2024. PMID: 38577173 Free PMC article.
-
Loss of MPC1 reprograms retinal metabolism to impair visual function.Proc Natl Acad Sci U S A. 2019 Feb 26;116(9):3530-3535. doi: 10.1073/pnas.1812941116. Epub 2019 Feb 11. Proc Natl Acad Sci U S A. 2019. PMID: 30808746 Free PMC article.
-
Aerobic Glycolysis Is Essential for Normal Rod Function and Controls Secondary Cone Death in Retinitis Pigmentosa.Cell Rep. 2018 May 29;23(9):2629-2642. doi: 10.1016/j.celrep.2018.04.111. Cell Rep. 2018. PMID: 29847794 Free PMC article.
-
Widespread Involvement of Acetylation in the Retinal Metabolism of Form-Deprivation Myopia in Guinea Pigs.ACS Omega. 2023 Jun 26;8(26):23825-23839. doi: 10.1021/acsomega.3c02219. eCollection 2023 Jul 4. ACS Omega. 2023. PMID: 37426266 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

