Analogous cellular contribution and healing mechanisms following digit amputation and phalangeal fracture in mice
- PMID: 27499878
- PMCID: PMC4857751
- DOI: 10.1002/reg2.51
Analogous cellular contribution and healing mechanisms following digit amputation and phalangeal fracture in mice
Abstract
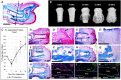
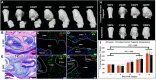
Regeneration of amputated structures is severely limited in humans and mice, with complete regeneration restricted to the distal portion of the terminal phalanx (P3). Here, we investigate the dynamic tissue repair response of the second phalangeal element (P2) post amputation in the adult mouse, and show that the repair response of the amputated bone is similar to the proximal P2 bone fragment in fracture healing. The regeneration-incompetent P2 amputation response is characterized by periosteal endochondral ossification resulting in the deposition of new trabecular bone, corresponding to a significant increase in bone volume; however, this response is not associated with bone lengthening. We show that cells of the periosteum respond to amputation and fracture by contributing both chondrocytes and osteoblasts to the endochondral ossification response. Based on our studies, we suggest that the amputation response represents an attempt at regeneration that ultimately fails due to the lack of a distal organizing influence that is present in fracture healing.
Keywords: Digit; endochondral ossification; fracture; mouse; periosteum; regeneration.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
