Cancer-testis antigens PRAME and NY-ESO-1 correlate with tumour grade and poor prognosis in myxoid liposarcoma
- PMID: 27499900
- PMCID: PMC4939879
- DOI: 10.1002/cjp2.16
Cancer-testis antigens PRAME and NY-ESO-1 correlate with tumour grade and poor prognosis in myxoid liposarcoma
Abstract
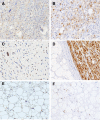
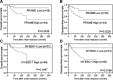
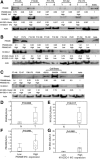
Myxoid liposarcoma is the second most common liposarcoma. Although myxoid liposarcoma is relatively chemosensitive and thus a good candidate for chemotherapy, cases with relapsed or metastatic disease still have poor outcome. Here, we performed a gene microarray analysis to compare the gene expression profiles in six clinical myxoid liposarcoma samples and three normal adipose tissue samples, and to identify molecular biomarkers that would be useful as diagnostic markers or treatment targets in myxoid liposarcoma. This showed that the cancer-testis antigen PRAME was up-regulated in myxoid liposarcoma. We then performed immunohistochemical, western blotting and real-time polymerase chain reaction analyses to quantify the expression of PRAME and another cancer-testis antigen, NY-ESO-1, in clinical samples of myxoid liposarcoma (n = 93), dedifferentiated (n = 46), well-differentiated (n = 32) and pleomorphic liposarcomas (n = 14). Immunohistochemically, positivity for PRAME and NY-ESO-1 was observed in 84/93 (90%) and 83/93 (89%) of the myxoid liposarcomas, and in 20/46 (43%) and 3/46 (7%) of the dedifferentiated, 3/32 (9%) and 1/32 (3%) of the well-differentiated and 7/14 (50%) and 3/21 (21%) of the pleomorphic liposarcomas, respectively. High immunohistochemical expression of PRAME and/or NY-ESO-1 was significantly correlated with tumour diameter, the existence of tumour necrosis, a round-cell component of >5%, higher histological grade and advanced clinical stage. High PRAME and NY-ESO-1 expression correlated significantly with poor prognosis in a univariate analysis. The myxoid liposarcomas showed significantly higher protein and mRNA expression levels of PRAME and NY-ESO-1 (CTAG1B) than the other liposarcomas. In conclusion, PRAME and NY-ESO-1 (CTAG1B) were expressed in the vast majority of myxoid liposarcomas, and their high-level expression correlated with tumour grade and poor prognosis. Our results support the potential use of PRAME and NY-ESO-1 as ancillary parameters for differential diagnosis and as prognostic biomarkers, and indicate that the development of immunotherapy against these cancer-testis antigens in myxoid liposarcoma would be warranted.
Keywords: NY‐ESO‐1; PRAME; cDNA microarray; cancer‐testis antigen; liposarcoma; myxoid liposarcoma.
Figures



Similar articles
-
The cancer-testis antigen NY-ESO-1 is highly expressed in myxoid and round cell subset of liposarcomas.Mod Pathol. 2013 Feb;26(2):282-8. doi: 10.1038/modpathol.2012.133. Epub 2012 Aug 31. Mod Pathol. 2013. PMID: 22936067
-
Cancer-testis antigen expression in synovial sarcoma: NY-ESO-1, PRAME, MAGEA4, and MAGEA1.Hum Pathol. 2017 Mar;61:130-139. doi: 10.1016/j.humpath.2016.12.006. Epub 2016 Dec 16. Hum Pathol. 2017. PMID: 27993576
-
NY-ESO-1 is a sensitive and specific immunohistochemical marker for myxoid and round cell liposarcomas among related mesenchymal myxoid neoplasms.Mod Pathol. 2013 Sep;26(9):1204-10. doi: 10.1038/modpathol.2013.65. Epub 2013 Apr 19. Mod Pathol. 2013. PMID: 23599152
-
The potential of the CMB305 vaccine regimen to target NY-ESO-1 and improve outcomes for synovial sarcoma and myxoid/round cell liposarcoma patients.Expert Rev Vaccines. 2018 Feb;17(2):107-114. doi: 10.1080/14760584.2018.1419068. Epub 2017 Dec 27. Expert Rev Vaccines. 2018. PMID: 29280411 Free PMC article. Review.
-
Managing Liposarcomas: Cutting Through the Fat.J Oncol Pract. 2016 Mar;12(3):221-7. doi: 10.1200/JOP.2015.009860. J Oncol Pract. 2016. PMID: 26962163 Review.
Cited by
-
Expression of Preferentially Expressed Antigen in Melanoma, a Cancer/Testis Antigen, in Carcinoma In Situ of the Urinary Tract.Diagnostics (Basel). 2023 Dec 10;13(24):3636. doi: 10.3390/diagnostics13243636. Diagnostics (Basel). 2023. PMID: 38132219 Free PMC article.
-
Comprehensive Analysis of a Six-Gene Signature Predicting Survival and Immune Infiltration of Liposarcoma Patients and Deciphering Its Therapeutic Significance.Int J Mol Sci. 2024 Jul 16;25(14):7792. doi: 10.3390/ijms25147792. Int J Mol Sci. 2024. PMID: 39063036 Free PMC article.
-
High prevalence of TERT aberrations in myxoid liposarcoma: TERT reactivation may play a crucial role in tumorigenesis.Cancer Sci. 2022 Mar;113(3):1078-1089. doi: 10.1111/cas.15256. Epub 2022 Jan 11. Cancer Sci. 2022. PMID: 34971481 Free PMC article.
-
[Expression of cancer testis (CT) antigens in pediatric and adolescent melanomas].Pathologe. 2017 Jul;38(4):303-311. doi: 10.1007/s00292-017-0311-z. Pathologe. 2017. PMID: 28631119 Review. German.
-
Preferentially expressed antigen in melanoma as a novel diagnostic marker differentiating thymic squamous cell carcinoma from thymoma.Sci Rep. 2020 Jul 23;10(1):12286. doi: 10.1038/s41598-020-69260-z. Sci Rep. 2020. PMID: 32704057 Free PMC article.
References
-
- Mack TM. Sarcomas and other malignancies of soft tissue, retroperitoneum, peritoneum, pleura, heart, mediastinum, and spleen. Cancer 1995; 1: 211–44. - PubMed
-
- Fletcher CDM, Bridge JA, Hogendoorn CWP, et al. WHO Classification of Tumor of Soft Tissue and Bone, (4th edn). IARC press: Lyon, 2013; 33–43.
-
- Moreau LC, Turcotte R, Ferguson P, et al. Myxoid/round cell liposarcoma (MRCLS) revisited: an analysis of 418 primarily managed cases. Ann Surg Oncol 2012; 19: 1081–1088. - PubMed
-
- Fiore M, Grosso F, Lo Vullo S, et al. Myxoid/round cell and pleomorphic liposarcomas: prognostic factors and survival in a series of patients treated at a single institution. Cancer 2007; 109: 2522–2531. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

