The Development of Fluorescence Intensity Standards
- PMID: 27500028
- PMCID: PMC4862808
- DOI: 10.6028/jres.106.015
The Development of Fluorescence Intensity Standards
Abstract
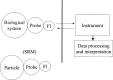
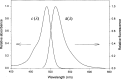
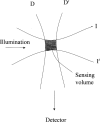
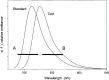
The use of fluorescence as an analytical technique has been growing over the last 20 years. A major factor in inhibiting more rapid growth has been the inability to make comparable fluorescence intensity measurements across laboratories. NIST recognizes the need to develop and provide primary fluorescence intensity standard (FIS) reference materials to the scientific and technical communities involved in these assays. The critical component of the effort will be the cooperation between the Federal laboratories, the manufacturers, and the technical personnel who will use the fluorescence intensity standards. We realize that the development and use of FIS will have to overcome many difficulties. However, as we outline in this article, the development of FIS is feasible.
Keywords: fluorescence intensity; quantitative fluorescence; standards.
Figures

References
-
- Marti G. History, Practical Theory and Consensus of Quantitative Flow Cytometry Measurements, Standards for QC/QA in Flow Cytometry. Bethesda, MD: CBER, FDA; 1997.
-
- Barker PE, Schwab M. Junction mapping of translocation chromosomes by fluorescence in situ hybridization (FISH) and computer image analysis in human solid tumors. Methods of Molecular Genetics. 1993;2:129–154.
-
- Schwartz A, Marti GE, Poon R, Gratama JW, Fernandez-Repollet E. Standardizing flow cytometry: A classification system of fluorescence standards used for flow cytometry. Cytometry. 1998;33:106–114. - PubMed
-
- Gratama JW, D’Hautcourt JL, Mandy F, Rothe G, Barnett D, Janossy G, Papa S, Schmitz G, Lenkei R. Flow cytometric quantitation of immunofluorescence intensity: Problems and perspectives. Cytometry. 1998;33:166–178. - PubMed
-
- Lenkei R, Gratama JW, Rothe G, Schmitz G, D’Hautcourt JL, Arekrans A, Mandy F, Marti G. Performance of calibration standards for antigen quantitation with flow cytometry. Cytometry. 1998;33:188–196. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
