Medium-Term Renal Function in a Large Cohort of Stable Kidney Transplant Recipients Converted From Twice-Daily to Once-Daily Tacrolimus
- PMID: 27500226
- PMCID: PMC4946473
- DOI: 10.1097/TXD.0000000000000536
Medium-Term Renal Function in a Large Cohort of Stable Kidney Transplant Recipients Converted From Twice-Daily to Once-Daily Tacrolimus
Abstract
Background: There is some evidence pointing toward better renal function in kidney transplant recipients (KTR) treated with once-daily tacrolimus (QD-TAC) vs. twice-daily tacrolimus (BID-TAC).
Methods: This is an extension study of a 1-year, single arm prospective study of stable KTR who were converted from BID-TAC to QD-TAC (4.9 ± 4.0 years after transplantation) in Spanish routine clinical practice. Patient and graft survival, renal function, acute rejection episodes, and other analytic parameters were assessed at 24 and 36 months after conversion.
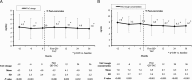
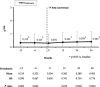
Results: A total of 1798 KTR were included in the extension study. Tacrolimus doses at 36 months were significantly lower compared to those at time of conversion (-0.2 mg/day; P = 0.023). Blood levels were lower than baseline during all the study (P < 0.001). Graft and patient survival at 3 years after conversion were 93.9% and 95.1%, respectively. Compared with baseline, the mean estimated glomerular filtration rate (eGFR) remained very stable at all timepoints (56.7 ± 19.8 vs 58.1 ± 24.6 mL/min per 1.73 m(2) at month 36; P = 0.623). Even when patients reinitiating dialysis were counted as eGFR = 0, the mean eGFR was very stable. In fact, a small but significant increase was observed at 36 months versus baseline (+0.1 mL/min per 1.73 m(2); P = 0.025). An increase in proteinuria was observed at 36 months versus baseline (+0.11 g/24 h; P < 0.001). Acute rejection rates were low during the study.
Conclusions: Conversion from BID-TAC to QD-TAC in a large cohort of stable KTR was safe and associated with a very stable renal function after 3 years. Comparative studies are warranted to assess the feasibility of such conversion.
Conflict of interest statement
The authors declare no funding or conflicts of interest.
L.G. participated in the research design, the performance of the research, the data analysis and the writing of the article. D.B. participated in the performance of the research and the writing of the article. C.C. participated in the performance of the research and the writing of the article. A.F. participated in the performance of the research and the writing of the article. A.F. participated in the performance of the research and the writing of the article. M.Á.G. participated in the performance of the research and the writing of the article. A.M. participated in the performance of the research and the writing of the article. J.V.T. participated in the performance of the research and the writing of the paper. E.G.H. participated in the performance of the research and the writing of the article. J.C.R. participated in the performance of the research and the writing of the article. J.S.P. participated in the performance of the research and the writing of the article. J.P. participated in the performance of the research and the writing of the article. R.L. participated in the performance of the research and the writing of the article. S.Z. participated in the performance of the research and the writing of the article. A.O. participated in the performance of the research and the writing of the article. C.J. participated in the performance of the research and the writing of the article. Á.A. participated in the performance of the research and the writing of the article. A.R. participated in the performance of the research and the writing of the article. B.B. participated in the data analysis and the writing of the article. D.H. participated in the performance of the research and the writing of the article.
Figures




References
-
- Chisholm MA, Middleton MD. Modified-release tacrolimus. Ann Pharmacother. 2006; 40: 270– 275. - PubMed
-
- Alloway R, Steinberg S, Khalil K, et al. Conversion of stable kidney transplant recipients from a twice daily Prograf-based regimen to a once daily modified release tacrolimus-based regimen. Transplant Proc. 2005; 37: 867– 870. - PubMed
-
- Silva HT, Jr, Yang HC, Abouljoud M, et al. One-year results with extended-release tacrolimus/MMF, tacrolimus/MMF and cyclosporine/MMF in de novo kidney transplant recipients. Am J Transplant. 2007; 7: 595– 608. - PubMed
-
- Krämer BK, Charpentier B, Bäckman L, et al. Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de novo renal transplantation: a randomized phase III study. Am J Transplant. 2010; 10: 2632– 2643. - PubMed
-
- Kuypers DR, Peeters PC, Sennesael JJ, et al. Improved adherence to tacrolimus once-daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation. 2013; 95: 333– 340. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

