Open-Label, Randomized Study of Transition From Tacrolimus to Sirolimus Immunosuppression in Renal Allograft Recipients
- PMID: 27500260
- PMCID: PMC4946511
- DOI: 10.1097/TXD.0000000000000579
Open-Label, Randomized Study of Transition From Tacrolimus to Sirolimus Immunosuppression in Renal Allograft Recipients
Abstract
Calcineurin inhibitor-associated nephrotoxicity and other adverse events have prompted efforts to minimize/eliminate calcineurin inhibitor use in kidney transplant recipients.
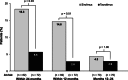
Methods: This open-label, randomized, multinational study evaluated the effect of planned transition from tacrolimus to sirolimus on kidney function in renal allograft recipients. Patients received tacrolimus-based immunosuppression and then were randomized 3 to 5 months posttransplantation to transition to sirolimus or continue tacrolimus. The primary end point was percentage of patients with 5 mL/min per 1.73 m(2) or greater improvement in estimated glomerular filtration rate from randomization to month 24.
Results: The on-therapy population included 195 patients (sirolimus, 86; tacrolimus, 109). No between-group difference was noted in percentage of patients with 5 mL/min per 1.73 m(2) or greater estimated glomerular filtration rate improvement (sirolimus, 34%; tacrolimus, 42%; P = 0.239) at month 24. Sirolimus patients had higher rates of biopsy-confirmed acute rejection (8% vs 2%; P = 0.02), treatment discontinuation attributed to adverse events (21% vs 3%; P < 0.001), and lower rates of squamous cell carcinoma of the skin (0% vs 5%; P = 0.012).
Conclusions: Our findings suggest that renal function improvement at 24 months is similar for patients with early conversion to sirolimus after kidney transplantation versus those remaining on tacrolimus.
Figures



Similar articles
-
Comparison of sirolimus plus tacrolimus versus sirolimus plus cyclosporine in high-risk renal allograft recipients: results from an open-label, randomized trial.Transplantation. 2008 Nov 15;86(9):1187-95. doi: 10.1097/TP.0b013e318187bab0. Transplantation. 2008. PMID: 19005398 Clinical Trial.
-
Renal function improves in liver transplant recipients when switched from a calcineurin inhibitor to sirolimus.Liver Transpl. 2003 Oct;9(10):1079-85. doi: 10.1053/jlts.2003.50183. Liver Transpl. 2003. PMID: 14526403 Clinical Trial.
-
A randomized controlled trial of late conversion from calcineurin inhibitor (CNI)-based to sirolimus-based immunosuppression in liver transplant recipients with impaired renal function.Liver Transpl. 2007 Dec;13(12):1694-702. doi: 10.1002/lt.21314. Liver Transpl. 2007. PMID: 18044728 Clinical Trial.
-
Tacrolimus: a further update of its use in the management of organ transplantation.Drugs. 2003;63(12):1247-97. doi: 10.2165/00003495-200363120-00006. Drugs. 2003. PMID: 12790696 Review.
-
Calcineurin inhibitor-sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity.Clin Transplant. 2008 Jan-Feb;22(1):1-15. doi: 10.1111/j.1399-0012.2007.00739.x. Clin Transplant. 2008. PMID: 18217899 Review.
Cited by
-
Immunomodulators for Non-Melanoma Skin Cancers: Updated Perspectives.Clin Cosmet Investig Dermatol. 2023 Apr 18;16:1025-1045. doi: 10.2147/CCID.S362171. eCollection 2023. Clin Cosmet Investig Dermatol. 2023. PMID: 37095898 Free PMC article. Review.
-
Current Biochemical Monitoring and Risk Management of Immunosuppressive Therapy after Transplantation.J Med Biochem. 2017 Jan 25;36(1):1-7. doi: 10.1515/jomb-2016-0029. eCollection 2017 Jan. J Med Biochem. 2017. PMID: 28680343 Free PMC article.
-
Reviewing 15 years of experience with sirolimus.Transplant Res. 2015 Dec 22;4(Suppl 1):6. doi: 10.1186/s13737-015-0028-6. eCollection 2015. Transplant Res. 2015. PMID: 27293553 Free PMC article. Review.
-
Safety and efficacy of Rapamune® (Sirolimus) in kidney transplant recipients: results of a prospective post-marketing surveillance study in Korea.BMC Nephrol. 2018 Aug 13;19(1):201. doi: 10.1186/s12882-018-1002-6. BMC Nephrol. 2018. PMID: 30103684 Free PMC article.
-
The "Personalising Actinic Keratosis Treatment for Immunocompromised Patients" (IM-PAKT) Project: An Expert Panel Opinion.Dermatol Ther (Heidelb). 2024 Jul;14(7):1739-1753. doi: 10.1007/s13555-024-01215-y. Epub 2024 Jun 21. Dermatol Ther (Heidelb). 2024. PMID: 38902589 Free PMC article. Review.
References
-
- Nankivell BJ, Borrows RJ, Fung CL, et al. The natural history of chronic allograft nephropathy. N Engl J Med. 2003; 349: 2326– 2333. - PubMed
-
- Hariharan S, McBride MA, Cherikh WS, et al. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int. 2002; 62: 311– 318. - PubMed
-
- Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009; 4: 481– 508. - PubMed
-
- Nankivell BJ, Borrows RJ, Fung CL, et al. Calcineurin inhibitor nephrotoxicity: longitudinal assessment by protocol histology. Transplantation. 2004; 78: 557– 565. - PubMed
-
- Lebranchu Y, Thierry A, Thervet E, et al. Efficacy and safety of early cyclosporine conversion to sirolimus with continued MMF—four-year results of the postconcept study. Am J Transplant. 2011; 11: 1665– 1675. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

