Glycoproteomic Analysis of Malignant Ovarian Cancer Ascites Fluid Identifies Unusual Glycopeptides
- PMID: 27500424
- PMCID: PMC5466807
- DOI: 10.1021/acs.jproteome.6b00548
Glycoproteomic Analysis of Malignant Ovarian Cancer Ascites Fluid Identifies Unusual Glycopeptides
Abstract
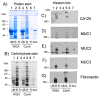
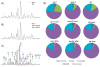
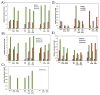
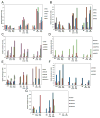
Ovarian cancer is a major cause of cancer mortality among women, largely due to late diagnosis of advanced metastatic disease. More extensive molecular analysis of metastatic ovarian cancer is needed to identify post-translational modifications of proteins, especially glycosylation that is particularly associated with metastatic disease to better understand the metastatic process and identify potential therapeutic targets. Glycoproteins in ascites fluid were enriched by affinity binding to lectins (ConA or WGA) and other affinity matrices. Separate glycomic, proteomic, and glycopeptide analyses were performed. Relative abundances of different N-glycan groups and proteins were identified from ascites fluids and a serum control. Levels of biomarkers CA125, MUC1, and fibronectin were also monitored in OC ascites samples by Western blot analysis. N-Glycan analysis of ascites fluids showed the presence of large, highly fucosylated and sialylated complex and hybrid glycans, some of which were not observed in normal serum. OC ascites glycoproteins, haptoglobin, fibronectin, lumican, fibulin, hemopexin, ceruloplasmin, alpha-1-antitrypsin, and alpha-1-antichymotrypsin were more abundant in OC ascites or not present in serum control samples. Further glycopeptide analysis of OC ascites identified N- and O-glycans in clusterin, hemopexin, and fibulin glycopeptides, some of which are unusual and may be important in OC metastasis.
Keywords: N-glycans; glycopeptides; glycoproteomics; malignant ascites; mass spectrometry; ovarian cancer; proteomics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Novel glycobiomarker for ovarian cancer that detects clear cell carcinoma.J Proteome Res. 2014 Mar 7;13(3):1624-35. doi: 10.1021/pr401109n. Epub 2014 Feb 14. J Proteome Res. 2014. PMID: 24498956
-
[Ascites May Provide Useful Information for Diagnosis of Ovarian Cancer].Klin Onkol. 2017 Spring;30(Supplementum1):187-190. Klin Onkol. 2017. PMID: 28471203 Czech.
-
The ascites N-glycome of epithelial ovarian cancer patients.J Proteomics. 2017 Mar 22;157:33-39. doi: 10.1016/j.jprot.2017.02.001. Epub 2017 Feb 8. J Proteomics. 2017. PMID: 28188862
-
Glycomics and glycoproteomics: Approaches to address isomeric separation of glycans and glycopeptides.J Sep Sci. 2021 Jan;44(1):403-425. doi: 10.1002/jssc.202000878. Epub 2020 Dec 14. J Sep Sci. 2021. PMID: 33090644 Review.
-
N-glycoproteomics in plants: perspectives and challenges.J Proteomics. 2011 Aug 12;74(8):1463-74. doi: 10.1016/j.jprot.2011.05.007. Epub 2011 May 12. J Proteomics. 2011. PMID: 21605711 Review.
Cited by
-
Understanding Ovarian Cancer: iTRAQ-Based Proteomics for Biomarker Discovery.Int J Mol Sci. 2018 Jul 31;19(8):2240. doi: 10.3390/ijms19082240. Int J Mol Sci. 2018. PMID: 30065196 Free PMC article.
-
Glycosylation and its research progress in endometrial cancer.Clin Transl Oncol. 2022 Oct;24(10):1865-1880. doi: 10.1007/s12094-022-02858-z. Epub 2022 Jun 25. Clin Transl Oncol. 2022. PMID: 35752750 Free PMC article. Review.
-
Multiple Reaction Monitoring for the Quantitation of Serum Protein Glycosylation Profiles: Application to Ovarian Cancer.J Proteome Res. 2018 Jan 5;17(1):222-233. doi: 10.1021/acs.jproteome.7b00541. Epub 2017 Dec 19. J Proteome Res. 2018. PMID: 29207246 Free PMC article.
-
Lectins: an effective tool for screening of potential cancer biomarkers.PeerJ. 2017 Sep 7;5:e3784. doi: 10.7717/peerj.3784. eCollection 2017. PeerJ. 2017. PMID: 28894650 Free PMC article.
-
In-Depth Mapping of the Urinary N-Glycoproteome: Distinct Signatures of ccRCC-related Progression.Cancers (Basel). 2020 Jan 18;12(1):239. doi: 10.3390/cancers12010239. Cancers (Basel). 2020. PMID: 31963743 Free PMC article.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. Ca-Cancer J Clin. 2013;63(1):11–30. - PubMed
-
- Kobayashi E, Ueda Y, Matsuzaki S, Yokoyama T, Kimura T, Yoshino K, Fujita M, Kimura T, Enomoto T. Biomarkers for screening, diagnosis, and monitoring of ovarian cancer. Cancer Epidemiol, Biomarkers Prev. 2012;21(11):1902–12. - PubMed
-
- Lu KH, Skates S, Hernandez MA, Bedi D, Bevers T, Leeds L, Moore R, Granai C, Harris S, Newland W, Adeyinka O, Geffen J, Deavers MT, Sun CC, Horick N, Fritsche H, Bast RC., Jr A 2-stage ovarian cancer screening strategy using the Risk of Ovarian Cancer Algorithm (ROCA) identifies early-stage incident cancers and demonstrates high positive predictive value. Cancer. 2013;119(19):3454–61. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

