An updated systematic review of epidemiological evidence on hormonal contraceptive methods and HIV acquisition in women
- PMID: 27500670
- PMCID: PMC5106090
- DOI: 10.1097/QAD.0000000000001228
An updated systematic review of epidemiological evidence on hormonal contraceptive methods and HIV acquisition in women
Abstract
Objective and design: Some studies suggest that specific hormonal contraceptive methods [particularly depot medroxyprogesterone acetate (DMPA)] may increase women's HIV acquisition risk. We updated a systematic review to incorporate recent epidemiological data.
Methods: We searched for articles published between 15 January 2014 and 15 January 2016 and hand-searched reference lists. We identified longitudinal studies comparing users of a specific hormonal contraceptive method against either nonusers of hormonal contraception or users of another specific hormonal contraceptive method. We added newly identified studies to those in the previous review, assessed study quality, created forest plots to display results, and conducted a meta-analysis for data on DMPA versus non-use of hormonal contraception.
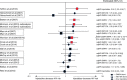
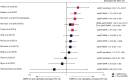
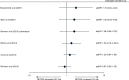
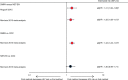
Results: We identified 10 new reports of which five were considered 'unlikely to inform the primary question'. We focus on the other five reports, along with nine from the previous review, which were considered 'informative but with important limitations'. The preponderance of data for oral contraceptive pills, injectable norethisterone enanthate, and levonorgestrel implants do not suggest an association with HIV acquisition, though data for implants are limited. The new, higher quality studies on DMPA (or nondisaggregated injectables), which had mixed results in terms of statistical significance, had hazard ratios between 1.2 and 1.7, consistent with our meta-analytic estimate for all higher quality studies of hazard ratio 1.4.
Conclusion: Although confounding in these observational data cannot be excluded, new information increases concerns about DMPA and HIV acquisition risk in women. If the association is causal, the magnitude of effect is likely hazard ratio 1.5 or less. Data for other hormonal contraceptive methods, including norethisterone enanthate, are largely reassuring.
Figures


Comment in
-
Levonorgestrel in contraceptives and multipurpose prevention technologies: does this progestin increase HIV risk or interact with antiretrovirals?AIDS. 2016 Nov 13;30(17):2571-2576. doi: 10.1097/QAD.0000000000001229. AIDS. 2016. PMID: 27525548 Free PMC article. No abstract available.
References
-
- Polis CB, Phillips SJ, Curtis KM, Westreich DJ, Steyn PS, Raymond E, et al. Hormonal contraceptive methods and risk of HIV acquisition in women: a systematic review of epidemiological evidence. Contraception 2014; 90:360–390. - PubMed
-
- Alkema L, Chou D, Hogan D, Zhang S, Moller AB, Gemmill A, et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet 2016; 387:462–474. - PMC - PubMed
-
- Schelar E, Polis CB, Essam T, Looker KJ, Bruni L, Chrisman CJ, et al. Multipurpose prevention technologies for sexual and reproductive health: mapping global needs for introduction of new preventive products. Contraception 2016; 93:32–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

