Control of Oocyte Reawakening by Kit
- PMID: 27500836
- PMCID: PMC4976968
- DOI: 10.1371/journal.pgen.1006215
Control of Oocyte Reawakening by Kit
Abstract
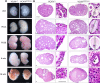
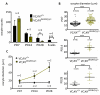
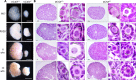
In mammals, females are born with finite numbers of oocytes stockpiled as primordial follicles. Oocytes are "reawakened" via an ovarian-intrinsic process that initiates their growth. The forkhead transcription factor Foxo3 controls reawakening downstream of PI3K-AKT signaling. However, the identity of the presumptive upstream cell surface receptor controlling the PI3K-AKT-Foxo3 axis has been questioned. Here we show that the receptor tyrosine kinase Kit controls reawakening. Oocyte-specific expression of a novel constitutively-active KitD818V allele resulted in female sterility and ovarian failure due to global oocyte reawakening. To confirm this result, we engineered a novel loss-of-function allele, KitL. Kit inactivation within oocytes also led to premature ovarian failure, albeit via a contrasting phenotype. Despite normal initial complements of primordial follicles, oocytes remained dormant with arrested oocyte maturation. Foxo3 protein localization in the nucleus versus cytoplasm explained both mutant phenotypes. These genetic studies provide formal genetic proof that Kit controls oocyte reawakening, focusing future investigations into the causes of primary ovarian insufficiency and ovarian aging.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- McGee E.A. and Hsueh A.J., Initial and cyclic recruitment of ovarian follicles. Endocr Rev, 2000. 21(2): p. 200–14. - PubMed
-
- Lintern-Moore S. and Moore G.P., The initiation of follicle and oocyte growth in the mouse ovary. Biol Reprod, 1979. 20(4): p. 773–8. - PubMed
-
- Matzuk M.M., et al., Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science, 2002. 296(5576): p. 2178–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

