Convergence of visual and whisker responses in the primary somatosensory thalamus (ventral posterior medial region) of the mouse
- PMID: 27501052
- PMCID: PMC5285619
- DOI: 10.1113/JP272791
Convergence of visual and whisker responses in the primary somatosensory thalamus (ventral posterior medial region) of the mouse
Abstract
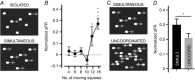
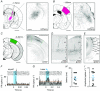
Key points: Using in vivo electrophysiology, we find that a subset of whisker-responsive neurons in the ventral posterior medial region (VPM) respond to visual stimuli. These light-responsive neurons in the VPM are particularly sensitive to optic flow. Presentation of optic flow stimuli modulates the amplitude of concurrent whisker responses. Visual information reaches the VPM via a circuit encompassing the visual cortex. These data represent a new example of cross-modal integration in the primary sensory thalamus.
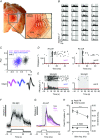
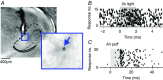
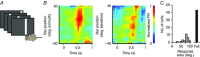
Abstract: Sensory signals reach the cortex via sense-specific thalamic nuclei. Here we report that neurons in the primary sensory thalamus of the mouse vibrissal system (the ventral posterior medial region; VPM) can be excited by visual as well as whisker stimuli. Using extracellular electrophysiological recordings from anaesthetized mice we first show that simple light steps can excite a subset of VPM neurons. We then test the ability of the VPM to respond to spatial patterns and show that many units are excited by visual motion in a direction-selective manner. Coherent movement of multiple objects (an artificial recreation of 'optic flow' that would usually occur during head rotations or body movements) best engages this visual motion response. We next show that, when co-applied with visual stimuli, the magnitude of responses to whisker deflections is highest in the presence of optic flow going in the opposite direction. Importantly, whisker response amplitude is also modulated by presentation of a movie recreating the mouse's visual experience during natural exploratory behaviour. We finally present functional and anatomical data indicating a functional connection (probably multisynaptic) from the primary visual cortex to VPM. These data provide a rare example of multisensory integration occurring at the level of the sensory thalamus, and provide evidence for dynamic regulation of whisker responses according to visual experience.
Keywords: VPM; multisensory; thalamus; vision.
© 2016 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures
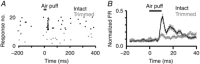




References
-
- Ahissar E, Sosnik R & Haidarliu S (2000). Transformation from temporal to rate coding in a somatosensory thalamocortical pathway. Nature 406, 302–306. - PubMed
-
- Berens P (2009). CircStat: a MATLAB toolbox for circular statistics. J Statist Software 31(10).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

