The prion protein is an agonistic ligand of the G protein-coupled receptor Adgrg6
- PMID: 27501152
- PMCID: PMC5499706
- DOI: 10.1038/nature19312
The prion protein is an agonistic ligand of the G protein-coupled receptor Adgrg6
Abstract
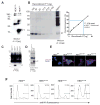
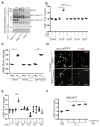
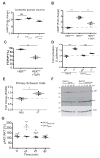
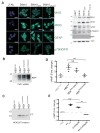
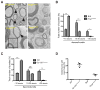
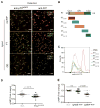
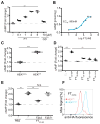
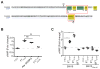
Ablation of the cellular prion protein PrP(C) leads to a chronic demyelinating polyneuropathy affecting Schwann cells. Neuron-restricted expression of PrP(C) prevents the disease, suggesting that PrP(C) acts in trans through an unidentified Schwann cell receptor. Here we show that the cAMP concentration in sciatic nerves from PrP(C)-deficient mice is reduced, suggesting that PrP(C) acts via a G protein-coupled receptor (GPCR). The amino-terminal flexible tail (residues 23-120) of PrP(C) triggered a concentration-dependent increase in cAMP in primary Schwann cells, in the Schwann cell line SW10, and in HEK293T cells overexpressing the GPCR Adgrg6 (also known as Gpr126). By contrast, naive HEK293T cells and HEK293T cells expressing several other GPCRs did not react to the flexible tail, and ablation of Gpr126 from SW10 cells abolished the flexible tail-induced cAMP response. The flexible tail contains a polycationic cluster (KKRPKPG) similar to the GPRGKPG motif of the Gpr126 agonist type-IV collagen. A KKRPKPG-containing PrPC-derived peptide (FT(23-50)) sufficed to induce a Gpr126-dependent cAMP response in cells and mice, and improved myelination in hypomorphic gpr126 mutant zebrafish (Danio rerio). Substitution of the cationic residues with alanines abolished the biological activity of both FT(23-50) and the equivalent type-IV collagen peptide. We conclude that PrP(C) promotes myelin homeostasis through flexible tail-mediated Gpr126 agonism. As well as clarifying the physiological role of PrP(C), these observations are relevant to the pathogenesis of demyelinating polyneuropathies--common debilitating diseases for which there are limited therapeutic options.
Conflict of interest statement
Figures




References
-
- Bremer J, et al. Axonal prion protein is required for peripheral myelin maintenance. Nat Neurosci. 2010;13:310–318. - PubMed
-
- Bueler H, et al. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582. - PubMed
-
- Kuwahara C, et al. Prions prevent neuronal cell-line death. Nature. 1999;400:225–226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

