Chronically Increased Amino Acids Improve Insulin Secretion, Pancreatic Vascularity, and Islet Size in Growth-Restricted Fetal Sheep
- PMID: 27501184
- PMCID: PMC5045508
- DOI: 10.1210/en.2016-1328
Chronically Increased Amino Acids Improve Insulin Secretion, Pancreatic Vascularity, and Islet Size in Growth-Restricted Fetal Sheep
Abstract
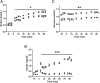
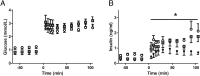
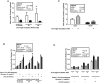
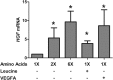
Placental insufficiency is associated with reduced supply of amino acids to the fetus and leads to intrauterine growth restriction (IUGR). IUGR fetuses are characterized by lower glucose-stimulated insulin secretion, smaller pancreatic islets with less β-cells, and impaired pancreatic vascularity. To test whether supplemental amino acids infused into the IUGR fetus could improve these complications of IUGR we used acute (hours) and chronic (11 d) direct fetal amino acid infusions into a sheep model of placental insufficiency and IUGR near the end of gestation. IUGR fetuses had attenuated acute amino acid-stimulated insulin secretion compared with control fetuses. These results were confirmed in isolated IUGR pancreatic islets. After the chronic fetal amino acid infusion, fetal glucose-stimulated insulin secretion and islet size were restored to control values. These changes were associated with normalization of fetal pancreatic vascularity and higher fetal pancreatic vascular endothelial growth factor A protein concentrations. These results demonstrate that decreased fetal amino acid supply contributes to the pathogenesis of pancreatic islet defects in IUGR. Moreover, the results show that pancreatic islets in IUGR fetuses retain their ability to respond to increased amino acids near the end of gestation after chronic fetal growth restriction.
Figures

References
-
- Van Assche FA, De Prins F, Aerts L, Verjans M. The endocrine pancreas in small-for-dates infants. Br J Obstet Gynaecol. 1977;84:751–753. - PubMed
-
- Nicolini U, Hubinont C, Santolaya J, Fisk NM, Rodeck CH. Effects of fetal intravenous glucose challenge in normal and growth retarded fetuses. Horm Metab Res. 1990;22:426–430. - PubMed
-
- Economides DL, Nicolaides KH, Linton EA, Perry LA, Chard T. Plasma cortisol and adrenocorticotropin in appropriate and small for gestational age fetuses. Fetal Ther. 1988;3:158–164. - PubMed
-
- Greenough A, Nicolaides KH, Lagercrantz H. Human fetal sympathoadrenal responsiveness. Early Hum Dev. 1990;23:9–13. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

