Germinal centre hypoxia and regulation of antibody qualities by a hypoxia response system
- PMID: 27501247
- PMCID: PMC5161594
- DOI: 10.1038/nature19334
Germinal centre hypoxia and regulation of antibody qualities by a hypoxia response system
Abstract
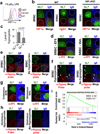
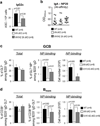
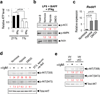
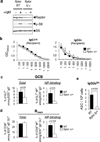
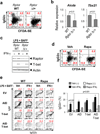
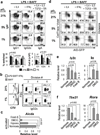
Germinal centres (GCs) promote humoral immunity and vaccine efficacy. In GCs, antigen-activated B cells proliferate, express high-affinity antibodies, promote antibody class switching, and yield B cell memory. Whereas the cytokine milieu has long been known to regulate effector functions that include the choice of immunoglobulin class, both cell-autonomous and extrinsic metabolic programming have emerged as modulators of T-cell-mediated immunity. Here we show in mice that GC light zones are hypoxic, and that low oxygen tension () alters B cell physiology and function. In addition to reduced proliferation and increased B cell death, low impairs antibody class switching to the pro-inflammatory IgG2c antibody isotype by limiting the expression of activation-induced cytosine deaminase (AID). Hypoxia induces HIF transcription factors by restricting the activity of prolyl hydroxyl dioxygenase enzymes, which hydroxylate HIF-1α and HIF-2α to destabilize HIF by binding the von Hippel-Landau tumour suppressor protein (pVHL). B-cell-specific depletion of pVHL leads to constitutive HIF stabilization, decreases antigen-specific GC B cells and undermines the generation of high-affinity IgG, switching to IgG2c, early memory B cells, and recall antibody responses. HIF induction can reprogram metabolic and growth factor gene expression. Sustained hypoxia or HIF induction by pVHL deficiency inhibits mTOR complex 1 (mTORC1) activity in B lymphoblasts, and mTORC1-haploinsufficient B cells have reduced clonal expansion, AID expression, and capacities to yield IgG2c and high-affinity antibodies. Thus, the normal physiology of GCs involves regional variegation of hypoxia, and HIF-dependent oxygen sensing regulates vital functions of B cells. We propose that the restriction of oxygen in lymphoid organs, which can be altered in pathophysiological states, modulates humoral immunity.
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

