Analysis of biophysical and functional consequences of tropomyosin-fluorescent protein fusions
- PMID: 27501521
- PMCID: PMC5053231
- DOI: 10.1002/1873-3468.12346
Analysis of biophysical and functional consequences of tropomyosin-fluorescent protein fusions
Abstract
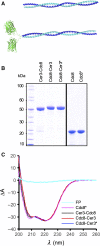
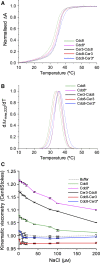
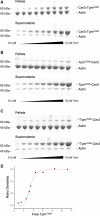
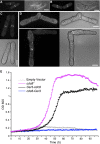
The dynamic nature of actin polymers is modulated to facilitate a diverse range of cellular processes. These dynamic properties are determined by different isoforms of tropomyosin which are recruited to distinct subpopulations of actin polymers to differentially regulate their functional properties. This makes tropomyosin an attractive target for labelling discrete actin populations. We have assessed the effect of different fluorescent labelling strategies for this protein. Although tropomyosin-fluorescent fusions decorate actin in vivo, they are either nonfunctional or perturb regulation of actin nucleation and cell cycle timings. Thus, conclusions and physiological relevance should be carefully evaluated when using tropomyosin fusions.
Keywords: Cdc8; Schizosaccharomyces pombe; acetylation; actin cytoskeleton; fission yeast.
© 2016 The Authors. FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures

References
-
- Pollard TD, Blanchoin L and Mullins RD (2000) Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu Rev Biophys Biomol Struct 29, 545–576. - PubMed
-
- Gunning PW, Schevzov G, Kee AJ and Hardeman EC (2005) Tropomyosin isoforms: divining rods for actin cytoskeleton function. Trends Cell Biol 15, 333–341. - PubMed
-
- Ebashi S and Ebashi F (1964) A new protein component participating in the superprecipitation of myosin B. J Biochem 55, 604–613. - PubMed
-
- Gunning PW, Hardeman EC, Lappalainen P and Mulvihill DP (2015) Tropomyosin – master regulator of actin filament function in the cytoskeleton. J Cell Sci 128, 2965–2974. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

