Telomeres and telomerase in head and neck squamous cell carcinoma: from pathogenesis to clinical implications
- PMID: 27501725
- PMCID: PMC5035656
- DOI: 10.1007/s10555-016-9633-1
Telomeres and telomerase in head and neck squamous cell carcinoma: from pathogenesis to clinical implications
Abstract
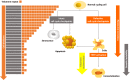
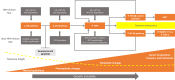
Strongly associated with tobacco use, heavy alcohol consumption, and with high-risk human papillomavirus (HPV) infection, head and neck squamous cell carcinoma (HNSCC) is a frequently lethal, heterogeneous disease whose pathogenesis is a multistep and multifactorial process involving genetic and epigenetic events. The majority of HNSCC patients present with locoregional advanced stage disease and are treated with combined modality strategies that can markedly impair quality of life and elicit unpredictable results. A large fraction of those who undergo locoregional treatment and achieve a complete response later develop locoregional recurrences or second field tumors. Biomarkers that are thus able to stratify risk and enable clinicians to tailor treatment plans and to personalize post-therapeutic surveillance strategies are highly desirable. To date, only HPV status is considered a reliable independent predictor of treatment response and survival in patients with HNSCC arising from the oropharyngeal site. Recent studies suggest that telomere attrition, which may be an early event in human carcinogenesis, and telomerase activation, which is detected in up to 90 % of malignancies, could be potential markers of cancer risk and disease outcome. This review examines the current state of knowledge on and discusses the implications linked to telomere dysfunction and telomerase activation in the development and clinical outcome of HNSCC.
Keywords: Field cancerization; Head and neck cancer; Human papillomavirus; Molecular biology; Recurrence; Survival; TERT; Telomerase reverse transcriptase; Telomere.
Conflict of interest statement
None to declare
Figures
References
-
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. European Journal of Cancer. 2013;49:1374–403. - PubMed
-
- Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nature Reviews Cancer. 2011;11:9–22. - PubMed
-
- Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. Journal of the National Cancer Institute. 2000;92:709–20. - PubMed
-
- Snijders PJ, Cromme FV, van den Brule AJ, Schrijnemakers HF, Snow GB, Meijer CJ, et al. Prevalence and expression of human papillomavirus in tonsillar carcinomas, indicating a possible viral etiology. International Journal of Cancer. 1992;51:845–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

