CXCR4 Overexpression in Human Adipose Tissue-Derived Stem Cells Improves Homing and Engraftment in an Animal Limb Ischemia Model
- PMID: 27501830
- PMCID: PMC5657758
- DOI: 10.3727/096368916X692708
CXCR4 Overexpression in Human Adipose Tissue-Derived Stem Cells Improves Homing and Engraftment in an Animal Limb Ischemia Model
Abstract
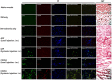
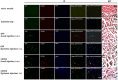
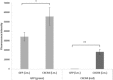
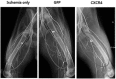
We investigated the effects of transplantation of CXCR4-overexpressing adipose tissue-derived stem cells (ADSCs) into a mouse diabetic hindlimb ischemia model on homing and engraftment as early as 48 h after transplant. CXCR4-overexpressing ADSCs were intramuscularly or intravenously injected into diabetic mice with hindlimb ischemia. After 48 h, muscle tissues in the femur and tibia were collected, and the CXCR4 expression pattern was analyzed by immunofluorescence staining. The homing and engraftment of transplanted CXCR4-overexpressing ADSCs into the ischemic area were significantly increased, and intravenous (systemic) injection resulted in the more effective delivery of stem cells to the target site 48 h posttransplantation. Furthermore, CXCR4-overexpressing ADSCs more efficiently contributed to long-term engraftment and muscle tissue regeneration than normal ADSCs in a limb ischemia model. In addition, the homing and engraftment of ADSCs were correlated with the CXCR4 transfection efficiency. These results demonstrated that enhanced CXCR4 signaling could significantly improve the early homing and engraftment of ADSCs into ischemic areas as well as the long-term engraftment and ultimate muscle tissue regeneration.
Figures





Similar articles
-
MRI of iron oxide nanoparticle-labeled ADSCs in a model of hindlimb ischemia.Biomaterials. 2013 Jul;34(21):4914-25. doi: 10.1016/j.biomaterials.2013.03.014. Epub 2013 Mar 25. Biomaterials. 2013. PMID: 23535037
-
Therapeutic Effect of Ligustilide-Stimulated Adipose-Derived Stem Cells in a Mouse Thromboembolic Stroke Model.Cell Transplant. 2016;25(5):899-912. doi: 10.3727/096368916X690539. Epub 2016 Jan 18. Cell Transplant. 2016. PMID: 26787228
-
Polymer-DNA Nanoparticle-Induced CXCR4 Overexpression Improves Stem Cell Engraftment and Tissue Regeneration in a Mouse Hindlimb Ischemia Model.Theranostics. 2016 May 23;6(8):1176-89. doi: 10.7150/thno.12866. eCollection 2016. Theranostics. 2016. PMID: 27279910 Free PMC article.
-
Update on the mechanisms of homing of adipose tissue-derived stem cells.Cytotherapy. 2016 Jul;18(7):816-27. doi: 10.1016/j.jcyt.2016.04.008. Cytotherapy. 2016. PMID: 27260205 Review.
-
Stromal-derived factor-1/CXCR4 signaling: indispensable role in homing and engraftment of hematopoietic stem cells in bone marrow.Stem Cells Dev. 2011 Jun;20(6):933-46. doi: 10.1089/scd.2010.0263. Epub 2011 Feb 2. Stem Cells Dev. 2011. PMID: 21186999 Review.
Cited by
-
A review of adipose-derived mesenchymal stem cells' impacts and challenges: metabolic regulation, tumor modulation, immunomodulation, regenerative medicine and genetic engineering therapies.Front Endocrinol (Lausanne). 2025 May 29;16:1606847. doi: 10.3389/fendo.2025.1606847. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40510466 Free PMC article. Review.
-
SDF-1 preconditioned HPC scaffolds mobilize cartilage-derived progenitors and stimulate meniscal fibrocartilage repair in human explant tissue culture.Connect Tissue Res. 2020 May-Jul;61(3-4):338-348. doi: 10.1080/03008207.2019.1689966. Epub 2019 Nov 19. Connect Tissue Res. 2020. PMID: 31744353 Free PMC article.
-
Current Strategies to Enhance Adipose Stem Cell Function: An Update.Int J Mol Sci. 2019 Aug 5;20(15):3827. doi: 10.3390/ijms20153827. Int J Mol Sci. 2019. PMID: 31387282 Free PMC article. Review.
-
Autologous Adipose-Derived Stem Cells Reduce Burn-Induced Neuropathic Pain in a Rat Model.Int J Mol Sci. 2017 Dec 22;19(1):34. doi: 10.3390/ijms19010034. Int J Mol Sci. 2017. PMID: 29271925 Free PMC article.
-
Pleiotropic Roles of CXCR4 in Wound Repair and Regeneration.Front Immunol. 2021 May 28;12:668758. doi: 10.3389/fimmu.2021.668758. eCollection 2021. Front Immunol. 2021. PMID: 34122427 Free PMC article. Review.
References
-
- Naderi AS, Farsian FN, Palmer BF. Diabetic muscle necrosis. J Diabetes Complications 2008; 22: 150–2. - PubMed
-
- Creager MA, Lüscher TF, Cosentino F, Beckman JA,. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 2003; 108: 1527–32. - PubMed
-
- Taniyama Y, Morishita R, Hiraoka K, Aoki M, Nakagami H, Yamasaki K, Matsumoto K, Nakamura T, Kaneda Y, Ogihara T. Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat diabetic hind limb ischemia model: Molecular mechanisms of delayed angiogenesis in diabetes. Circulation 2001; 104: 2344–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

