Nanoroughened adhesion-based capture of circulating tumor cells with heterogeneous expression and metastatic characteristics
- PMID: 27501846
- PMCID: PMC4977622
- DOI: 10.1186/s12885-016-2638-x
Nanoroughened adhesion-based capture of circulating tumor cells with heterogeneous expression and metastatic characteristics
Abstract
Background: Circulating tumor cells (CTCs) have shown prognostic relevance in many cancer types. However, the majority of current CTC capture methods rely on positive selection techniques that require a priori knowledge about the surface protein expression of disseminated CTCs, which are known to be a dynamic population.
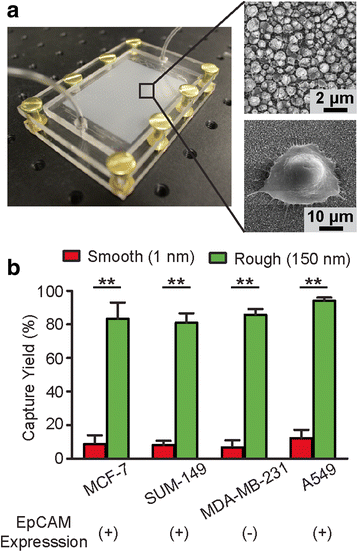
Methods: We developed a microfluidic CTC capture chip that incorporated a nanoroughened glass substrate for capturing CTCs from blood samples. Our CTC capture chip utilized the differential adhesion preference of cancer cells to nanoroughened etched glass surfaces as compared to normal blood cells and thus did not depend on the physical size or surface protein expression of CTCs.
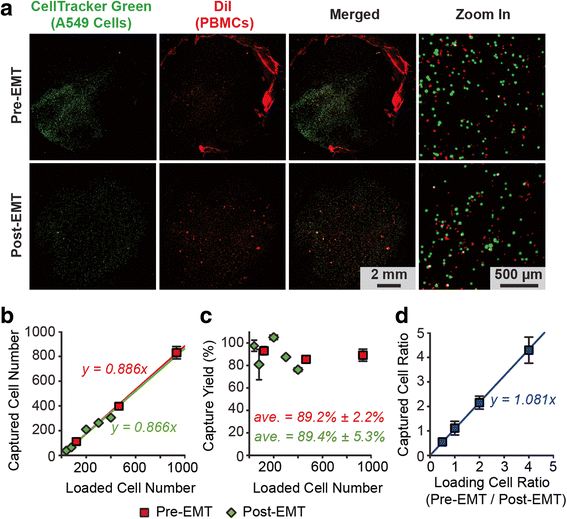
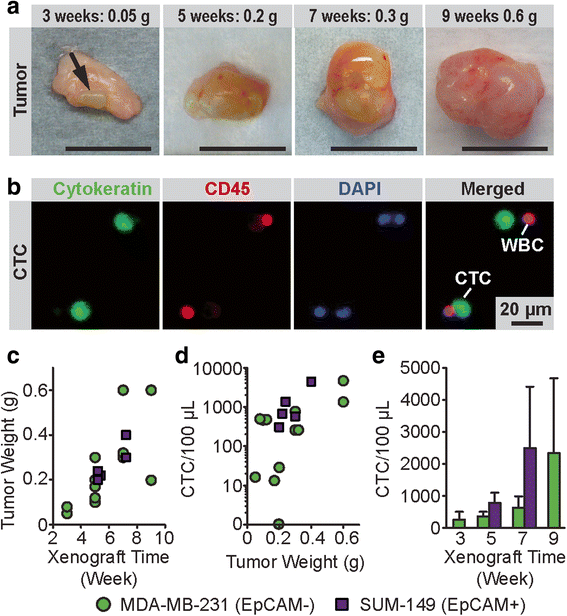
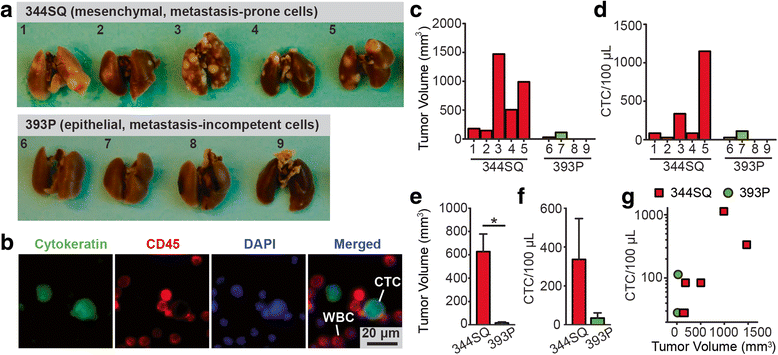
Results: The microfluidic CTC capture chip was able to achieve a superior capture yield for both epithelial cell adhesion molecule positive (EpCAM+) and EpCAM- cancer cells in blood samples. Additionally, the microfluidic CTC chip captured CTCs undergoing transforming growth factor beta-induced epithelial-to-mesenchymal transition (TGF-β-induced EMT) with dynamically down-regulated EpCAM expression. In a mouse model of human breast cancer using EpCAM positive and negative cell lines, the number of CTCs captured correlated positively with the size of the primary tumor and was independent of their EpCAM expression. Furthermore, in a syngeneic mouse model of lung cancer using cell lines with differential metastasis capability, CTCs were captured from all mice with detectable primary tumors independent of the cell lines' metastatic ability.
Conclusions: The microfluidic CTC capture chip using a novel nanoroughened glass substrate is broadly applicable to capturing heterogeneous CTC populations of clinical interest independent of their surface marker expression and metastatic propensity. We were able to capture CTCs from a non-metastatic lung cancer model, demonstrating the potential of the chip to collect the entirety of CTC populations including subgroups of distinct biological and phenotypical properties. Further exploration of the biological potential of metastatic and presumably non-metastatic CTCs captured using the microfluidic chip will yield insights into their relevant differences and their effects on tumor progression and cancer outcomes.
Keywords: Adhesion; Breast cancer; Circulating tumor cells; Lung cancer; Metastasis; Microfluidics.
Figures
Similar articles
-
EpCAM-independent capture of circulating tumor cells with a 'universal CTC-chip'.Oncol Rep. 2017 Jan;37(1):77-82. doi: 10.3892/or.2016.5235. Epub 2016 Nov 8. Oncol Rep. 2017. PMID: 27840987
-
Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition.BMC Cancer. 2012 May 16;12:178. doi: 10.1186/1471-2407-12-178. BMC Cancer. 2012. PMID: 22591372 Free PMC article.
-
Prognostic impact of circulating tumor cells detected with the microfluidic "universal CTC-chip" for primary lung cancer.Cancer Sci. 2022 Mar;113(3):1028-1037. doi: 10.1111/cas.15255. Epub 2022 Jan 28. Cancer Sci. 2022. PMID: 34964211 Free PMC article.
-
Multifaceted Approaches in Epithelial Cell Adhesion Molecule-Mediated Circulating Tumor Cell Isolation.Molecules. 2025 Feb 20;30(5):976. doi: 10.3390/molecules30050976. Molecules. 2025. PMID: 40076201 Free PMC article. Review.
-
Epithelial Cell Adhesion Molecule: An Anchor to Isolate Clinically Relevant Circulating Tumor Cells.Cells. 2020 Aug 5;9(8):1836. doi: 10.3390/cells9081836. Cells. 2020. PMID: 32764280 Free PMC article. Review.
Cited by
-
Role of Cell Adhesion in Cancer Metastasis Formation: A Review.ACS Omega. 2025 Feb 9;10(6):5193-5213. doi: 10.1021/acsomega.4c08140. eCollection 2025 Feb 18. ACS Omega. 2025. PMID: 39989825 Free PMC article. Review.
-
Phenotypic Characterization of Circulating Lung Cancer Cells for Clinically Actionable Targets.Cancers (Basel). 2019 Mar 18;11(3):380. doi: 10.3390/cancers11030380. Cancers (Basel). 2019. PMID: 30889898 Free PMC article.
-
Effective capture of circulating tumor cells from an S180-bearing mouse model using electrically charged magnetic nanoparticles.J Nanobiotechnology. 2019 May 4;17(1):59. doi: 10.1186/s12951-019-0491-1. J Nanobiotechnology. 2019. PMID: 31054582 Free PMC article.
-
Microfluidics for studying metastatic patterns of lung cancer.J Nanobiotechnology. 2019 May 27;17(1):71. doi: 10.1186/s12951-019-0492-0. J Nanobiotechnology. 2019. PMID: 31133019 Free PMC article. Review.
-
Conquering the challenges of genotypic and phenotypic tumor heterogeneity to realize the promise of personalized cancer therapy: the role of academia.Trans Am Clin Climatol Assoc. 2017;128:169-179. Trans Am Clin Climatol Assoc. 2017. PMID: 28790501 Free PMC article.
References
-
- Ashworth T. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J. 1869;14:146–9.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

