Fetal Tissues Tested for Microbial Sterility by Culture- and PCR-Based Methods Can be Safely Used in Clinics
- PMID: 27501947
- PMCID: PMC5657759
- DOI: 10.3727/096368916X692735
Fetal Tissues Tested for Microbial Sterility by Culture- and PCR-Based Methods Can be Safely Used in Clinics
Abstract
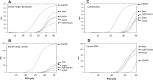
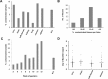
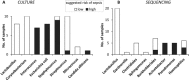
Cell preparations to be used in clinical practice must be free of infectious agents. Safety concerns are especially elevated upon the use of human fetal tissues, which are otherwise highly advantageous in cell therapy. We demonstrate that treating fetal samples with antibiotic, extensive washing, and homogenization prior to cryoconservation efficiently removes microbes in general. Screening a large collection by an automatic culture system showed that 89.2% fetal tissue samples were sterile, while contamination was detected in 10.8% samples. Liver and chorion were contaminated more than the brain, kidney, lung, and soft tissues. Broad-range PCR from the bacterial 16s rRNA gene was adopted as a confirmatory assay; however, the concordance between the culture-based and PCR assays was weak. Taxonomic identification was done for contaminated samples by bacteriological methods and sequencing 16s rRNA PCR products. The two approaches revealed different spectra of taxonomic groups sharing only Lactobacillus, the most frequently found genus. In addition, other representatives of vaginal microbiota were detected by culture-based identification, while PCR product sequencing has also revealed a subset of nosocomial microorganisms. Importantly, species known to cause sepsis were identified by both techniques, arguing for their indispensability and mutual complementarity. We suggest that most contaminations are taken up during collection of fetal material rather than originating from an in utero infection. In conclusion, a rigorous microbiological control by culture and PCR is a prerequisite for safe clinical use of fetal tissue suspensions.
Figures


Similar articles
-
Broad-range 16S rRNA gene polymerase chain reaction for diagnosis of culture-negative bacterial infections.Clin Infect Dis. 2011 Dec;53(12):1245-51. doi: 10.1093/cid/cir692. Epub 2011 Oct 5. Clin Infect Dis. 2011. PMID: 21976460
-
Fetal environment and fetal intestine are sterile during the third trimester of pregnancy.Vet Immunol Immunopathol. 2018 Oct;204:59-64. doi: 10.1016/j.vetimm.2018.09.005. Epub 2018 Sep 26. Vet Immunol Immunopathol. 2018. PMID: 30290960
-
Pathogen Identification in Suspected Cases of Pyogenic Spondylodiscitis.Front Cell Infect Microbiol. 2017 Mar 9;7:60. doi: 10.3389/fcimb.2017.00060. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28337426 Free PMC article.
-
[Species and strain specific identification of lactic acid bacteria in complex microflora].Berl Munch Tierarztl Wochenschr. 2003 Nov-Dec;116(11-12):517-23. Berl Munch Tierarztl Wochenschr. 2003. PMID: 14655632 Review. German.
-
Use of broad range16S rDNA PCR in clinical microbiology.J Microbiol Methods. 2009 Mar;76(3):217-25. doi: 10.1016/j.mimet.2008.11.002. Epub 2008 Nov 21. J Microbiol Methods. 2009. PMID: 19046999 Review.
Cited by
-
Microbial occurrence in liquid nitrogen storage tanks: a challenge for cryobanking?Appl Microbiol Biotechnol. 2021 Oct;105(20):7635-7650. doi: 10.1007/s00253-021-11531-4. Epub 2021 Sep 24. Appl Microbiol Biotechnol. 2021. PMID: 34559283 Free PMC article. Review.
-
Prevalence of human pegivirus-1 and sequence variability of its E2 glycoprotein estimated from screening donors of fetal stem cell-containing material.Virol J. 2017 Aug 31;14(1):167. doi: 10.1186/s12985-017-0837-y. Virol J. 2017. PMID: 28859680 Free PMC article.
References
-
- Bhattacharya N. Fetal cell/tissue therapy in adult disease: A new horizon in regenerative medicine. Clin Exp Obstet Gynecol. 2004; 31(3): 167–73. - PubMed
-
- Bradstreet JJ, Sych N, Antonucci N, Klunnik M, Ivankova O, Matyashchuk I, Demchuk M, Siniscalco D. Efficacy of fetal stem cell transplantation in autism spectrum disorders: An open-labeled pilot study. Cell Transplant. 2014; 23(suppl 1): S105–12. - PubMed
-
- Madrazo I, León V, Torres C, Aguilera MC, Varela G, Alvarez F, Fraga A, Drucker-Colín R, Ostrosky F, Skurovich M,. Transplantation of fetal substantia nigra and adrenal medulla to the caudate nucleus in two patients with Parkinson's disease. N Engl J Med. 1988; 318(1): 51. - PubMed
-
- Benetti F, Peñherrera E, Maldonado T, Vera YD, Subramanian V, Geffner L,. Direct myocardial implantation of human fetal stem cells in heart failure patients: Long-term results. Heart Surg Forum 2010; 13(1): E31–5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

