The influence hydrogen atom addition has on charge switching during motion of the metal atom in endohedral Ca@C60H4 isomers
- PMID: 27501967
- PMCID: PMC4978743
- DOI: 10.1098/rsta.2015.0319
The influence hydrogen atom addition has on charge switching during motion of the metal atom in endohedral Ca@C60H4 isomers
Abstract
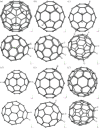
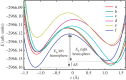
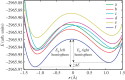
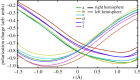
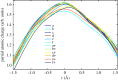
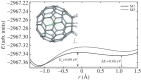
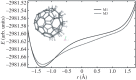
Density functional theory has been applied in a study of charge transfer between an endohedral calcium atom and the fullerene cage in Ca@C60H4 and [Ca@C60H4](+) isomers. Previous calculations on Ca@C60 have shown that the motion of calcium within a fullerene is accompanied by large changes in electron density on the carbon cage. Based on this observation, it has been proposed that a tethered endohedral fullerene might form the bases of a nanoswitch. Through the addition of hydrogen atoms to one hemisphere of the cage it is shown that, when compared with Ca@C60, asymmetric and significantly reduced energy barriers can be generated with respect to motion of the calcium atom. It is proposed that hydrogen atom addition to a fullerene might offer a route for creating a bi-stable nanoswitch that can be fine-tuned through the selection of an appropriate isomer and number of atoms attached to the cage of an endohedral fullerene.This article is part of the themed issue 'Fullerenes: past, present and future, celebrating the 30th anniversary of Buckminster Fullerene'.
Keywords: calcium; density functional theory; endohedral; fullerene; nanoswitch.
© 2016 The Author(s).
Figures



Similar articles
-
Polarisation charge switching through the motion of metal atoms trapped in fullerene cages.Phys Chem Chem Phys. 2014 Nov 21;16(43):23869-73. doi: 10.1039/c4cp02672a. Epub 2014 Oct 2. Phys Chem Chem Phys. 2014. PMID: 25272966
-
Super-atom molecular orbital excited states of fullerenes.Philos Trans A Math Phys Eng Sci. 2016 Sep 13;374(2076):20150322. doi: 10.1098/rsta.2015.0322. Philos Trans A Math Phys Eng Sci. 2016. PMID: 27501970 Free PMC article. Review.
-
Fullerene ion chemistry: a journey of discovery and achievement.Philos Trans A Math Phys Eng Sci. 2016 Sep 13;374(2076):20150321. doi: 10.1098/rsta.2015.0321. Philos Trans A Math Phys Eng Sci. 2016. PMID: 27501972 Free PMC article. Review.
-
Ab initio infrared vibrational modes for neutral and charged small fullerenes (C20, C24, C26, C28, C30 and C60).Philos Trans A Math Phys Eng Sci. 2016 Sep 13;374(2076):20150323. doi: 10.1098/rsta.2015.0323. Philos Trans A Math Phys Eng Sci. 2016. PMID: 27501975 Free PMC article.
-
Atom-Cage Charge Transfer in Endohedral Metallofullerenes: Trapping Atoms Within a Sphere-Like Ridge of Avoided Crossings.J Phys Chem Lett. 2013 Feb 7;4(3):422-5. doi: 10.1021/jz3020259. Epub 2013 Jan 16. J Phys Chem Lett. 2013. PMID: 26281734
Cited by
-
Recent Developments in the Methods and Applications of Electrostatic Theory.Acc Chem Res. 2023 Sep 5;56(17):2267-2277. doi: 10.1021/acs.accounts.3c00068. Epub 2023 Aug 16. Acc Chem Res. 2023. PMID: 37585560 Free PMC article.
References
-
- Shinohara H. 2000. Endohedral metallofullerenes. Rep. Prog. Phys. 63, 843–892. (10.1088/0034-4885/63/6/201) - DOI
-
- Raehm L, Sauvage J-P. 2001. Molecular machines and motors based on transition metal-containing catenanes and rotaxanes. In Molecular machines and motors (eds J-P Sauvage et al.). Structure and Bonding, vol. 99, pp. 55–78. Berlin, Germany: Springer. ()
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

