Fullerene ion chemistry: a journey of discovery and achievement
- PMID: 27501972
- PMCID: PMC4978745
- DOI: 10.1098/rsta.2015.0321
Fullerene ion chemistry: a journey of discovery and achievement
Abstract
An account is provided of the extraordinary features of buckminster fullerene cations and their chemistry that we discovered in our Ion Chemistry Laboratory at York University (Canada) during a 'golden' period of research in the early 1990s, just after C60 powder became available. We identified new chemical ways of C60 ionization and tracked novel chemistry of C60 (n+) as a function of charge state (n=1-3) with some 50 different reagent molecules. We found that multiple charges enhance reaction rates and diversify reaction products and mechanisms. Strong electrostatic interactions with reagent molecules were seen to reduce barriers to carbon surface bonding and charge-separation reactions, while intramolecular Coulomb repulsion appeared to localize charge on the surface or the substituent and so influence higher order chemistry, including 'spindle', 'star', 'fuzzy ball', 'ball-and-chain' and dimer ion formation. We introduced the notion of 'apparent' gas-phase acidity with measurements of proton-transfer reactions of multiply charged fullerene cations. We also explored the attachment of atomic metal cations to C60 and their subsequent reactions. All these findings were applied to the possible chemistry of fullerene cations in the interstellar medium with a focus on multiply charged fullerene ion formation and the intervention of fullerene cations in fullerene derivatization and molecular synthesis, with a view to their possible future detection.This article is part of the themed issue 'Fullerenes: past, present and future, celebrating the 30th anniversary of Buckminster Fullerene'.
Keywords: buckminster fullerene cations; charge state chemistry; electron transfer; extraterrestrial chemistry.
© 2016 The Author(s).
Figures


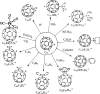



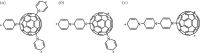

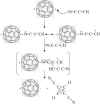
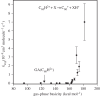
 at the onset of proton transfer.
at the onset of proton transfer.
Similar articles
-
Pathway to the identification of C60+ in diffuse interstellar clouds.Philos Trans A Math Phys Eng Sci. 2016 Sep 13;374(2076):20150316. doi: 10.1098/rsta.2015.0316. Philos Trans A Math Phys Eng Sci. 2016. PMID: 27501976 Free PMC article. Review.
-
The influence hydrogen atom addition has on charge switching during motion of the metal atom in endohedral Ca@C60H4 isomers.Philos Trans A Math Phys Eng Sci. 2016 Sep 13;374(2076):20150319. doi: 10.1098/rsta.2015.0319. Philos Trans A Math Phys Eng Sci. 2016. PMID: 27501967 Free PMC article.
-
Multiply-charged ions and interstellar chemistry.Phys Chem Chem Phys. 2011 Nov 7;13(41):18253-63. doi: 10.1039/c1cp21814j. Epub 2011 Aug 25. Phys Chem Chem Phys. 2011. PMID: 21869973
-
Ab initio infrared vibrational modes for neutral and charged small fullerenes (C20, C24, C26, C28, C30 and C60).Philos Trans A Math Phys Eng Sci. 2016 Sep 13;374(2076):20150323. doi: 10.1098/rsta.2015.0323. Philos Trans A Math Phys Eng Sci. 2016. PMID: 27501975 Free PMC article.
-
Ion/ion chemistry of high-mass multiply charged ions.Mass Spectrom Rev. 1998 Nov-Dec;17(6):369-407. doi: 10.1002/(SICI)1098-2787(1998)17:6<369::AID-MAS1>3.0.CO;2-J. Mass Spectrom Rev. 1998. PMID: 10360331 Review.
Cited by
-
Unraveling the Metastability of C n2+ ( n = 2-4) Clusters.J Phys Chem Lett. 2019 Feb 7;10(3):581-588. doi: 10.1021/acs.jpclett.8b03449. Epub 2019 Jan 24. J Phys Chem Lett. 2019. PMID: 30673242 Free PMC article.
-
Ion spectroscopy in methane activation.Mass Spectrom Rev. 2022 Jul;41(4):513-528. doi: 10.1002/mas.21698. Epub 2021 May 19. Mass Spectrom Rev. 2022. PMID: 34008884 Free PMC article. Review.
References
-
- Kroto HW, Heath JR, O’Brian SC, Curl RF, Smalley RE. 1985. C60: Buckminsterfullerene. Nature 318, 162–163. (10.1038/318162a0) - DOI
-
- Mackay GI, Vlachos GD, Bohme DK, Schiff HI. 1980. Studies of reactions involving C2H+X ions with HCN using a modified selected ion flow tube. J. Mass Spectrom. Ion Phys. 36, 259–270. (10.1016/0020-7381(80)85059-5) - DOI
-
- Krätschmer W, Lamb LD, Fostiropoulis K, Huffman DR. 1990. Solid C60: a new form of carbon. Nature 347, 354–358. (10.1038/347354a0) - DOI
-
- Böhme DK. 1999. 1998 J.C. Polanyi Award Lecture. Fullerene ions in the gas phase: chemistry as a function of charge state. Can. J. Chem. 77, 1453–1464. (10.1139/v99-158) - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

