p-Hydroxylcinnamaldehyde induces the differentiation of oesophageal carcinoma cells via the cAMP-RhoA-MAPK signalling pathway
- PMID: 27501997
- PMCID: PMC4977536
- DOI: 10.1038/srep31315
p-Hydroxylcinnamaldehyde induces the differentiation of oesophageal carcinoma cells via the cAMP-RhoA-MAPK signalling pathway
Abstract
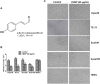
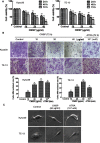
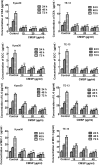
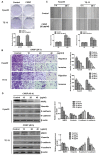
p-Hydroxylcinnamaldehyde (CMSP) has been identified as an inhibitor of the growth of various cancer cells. However, its function in oesophageal squamous cell carcinoma (ESCC) and the underlying mechanism remain unclear. The aim of the present study was to characterize the differentiation effects of CMSP, as well as its mechanism in the differentiation of ESCC Kyse30 and TE-13 cells. The function of CMSP in the viability, colony formation, migration and invasion of Kyse30 and TE-13 cells was determined by MTS, colony-formation, wound healing and transwell assays. Western blotting and pull-down assays were used to investigate the effect of CMSP on the expression level of malignant markers of ESCC, as well as the activity of MAPKs, RhoA and GTP-RhoA in Kyse30 and TE-13 cells. We found that CMSP could inhibit proliferation and migration and induce Kyse30 and TE-13 cell differentiation, characterized by dendrite-like outgrowth, decreased expression of tumour-associated antigens, as well as the decreased expression of malignant markers. Furthermore, increased cAMP, p-P38 and decreased activities of ERK, JNK and GTP-RhoA, were detected after treatment with CMSP. These results indicated that CMSP induced the differentiation of Kyse30 and TE-13 cells through mediating the cAMP-RhoA-MAPK axis, which might provide new potential strategies for ESCC treatment.
Figures

Similar articles
-
p-Hydroxylcinnamaldehyde slows the progression of 4NQO-induced oesophageal tumourigenesis via the RhoA-MAPK signaling pathway.Mol Carcinog. 2018 Oct;57(10):1319-1331. doi: 10.1002/mc.22847. Epub 2018 Jun 16. Mol Carcinog. 2018. PMID: 29873419
-
p-Hydroxylcinnamaldehyde from cochinchinamomordica seed reverses resistance to TRAIL in human oesophageal squamous cell carcinoma via the activation of the p38 mitogen-activated protein kinase signalling pathway.Biomed Pharmacother. 2020 Jan;121:109611. doi: 10.1016/j.biopha.2019.109611. Epub 2019 Nov 12. Biomed Pharmacother. 2020. PMID: 31731196
-
P-Hydroxycinnamaldehyde Induces B16-F1 Melanoma Cell Differentiation via the RhoA-MAPK Signaling Pathway.Cell Physiol Biochem. 2016;38(6):2247-60. doi: 10.1159/000445580. Epub 2016 May 19. Cell Physiol Biochem. 2016. PMID: 27188168
-
3,3'-Diindolylmethane modulates aryl hydrocarbon receptor of esophageal squamous cell carcinoma to reverse epithelial-mesenchymal transition through repressing RhoA/ROCK1-mediated COX2/PGE2 pathway.J Exp Clin Cancer Res. 2020 Jun 16;39(1):113. doi: 10.1186/s13046-020-01618-7. J Exp Clin Cancer Res. 2020. PMID: 32546278 Free PMC article.
-
RhoA and RhoC proteins promote both cell proliferation and cell invasion of human oesophageal squamous cell carcinoma cell lines in vitro and in vivo.Eur J Cancer. 2006 Jul;42(10):1455-65. doi: 10.1016/j.ejca.2006.02.012. Epub 2006 Jun 5. Eur J Cancer. 2006. PMID: 16750623
Cited by
-
ERK is a Pivotal Player of Chemo-Immune-Resistance in Cancer.Int J Mol Sci. 2019 May 21;20(10):2505. doi: 10.3390/ijms20102505. Int J Mol Sci. 2019. PMID: 31117237 Free PMC article. Review.
-
CMSP exerts anti-tumor effects on small cell lung cancer cells by inducing mitochondrial dysfunction and ferroptosis.Open Med (Wars). 2025 Jan 15;20(1):20241100. doi: 10.1515/med-2024-1100. eCollection 2025. Open Med (Wars). 2025. PMID: 39822985 Free PMC article.
-
Momordica cochinchinensis (Gấc) Seed Extracts Induce Apoptosis and Necrosis in Melanoma Cells.Pharmaceuticals (Basel). 2023 Jan 9;16(1):100. doi: 10.3390/ph16010100. Pharmaceuticals (Basel). 2023. PMID: 36678596 Free PMC article.
-
Knockdown of FAM83D Enhances Radiosensitivity in Coordination with Irradiation by Inhibiting EMT via the Akt/GSK-3β/Snail Signaling Pathway in Human Esophageal Cancer Cells.Onco Targets Ther. 2020 May 26;13:4665-4678. doi: 10.2147/OTT.S245681. eCollection 2020. Onco Targets Ther. 2020. PMID: 32547096 Free PMC article.
-
ADAMTS8 targets ERK to suppress cell proliferation, invasion, and metastasis of hepatocellular carcinoma.Onco Targets Ther. 2018 Oct 29;11:7569-7578. doi: 10.2147/OTT.S173360. eCollection 2018. Onco Targets Ther. 2018. PMID: 30464505 Free PMC article.
References
-
- Cheng W. Q. et al.. Cancer Statistics in China, 2015. Ca Cancer J Clin 66, 115–132 (2016). - PubMed
-
- Zhao L. M. et al.. An ester extract of Cochinchinamomordica seeds induces differentiation of melanoma B16 F1 cells via MAPKs signaling. Asian Pac J Cancer Prev 13, 3795–3802 (2012). - PubMed
-
- Lu T. Y. et al.. Inhibition effects of all trans-retinoic acid on the growth and angiogenesis of esophagealsquamous cell carcinoma in nude mice. Chin Med J (Engl) 124, 2708–2714 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

