A simultaneous assessment metric for MAb quantity and glycan quality
- PMID: 27502107
- PMCID: PMC5023566
- DOI: 10.1007/s10616-016-0011-1
A simultaneous assessment metric for MAb quantity and glycan quality
Abstract
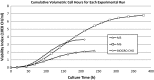
As a critical quality attribute, glycosylation represents an important consideration when analyzing the success of a glycoprotein production process. Though critical, glycosylation is not the only measure of culture success; other factors, including culture size, maintenance, and productivity, are also critical. A new metric was developed to address both product quality, as measured through glycosylation, and product quantity, as measured through product concentration. A monoclonal antibody Chinese hamster ovary cell culture model system was used to assess this metric across various media formulations. In a model test system, the metric discriminated that some media supplements had a net positive impact on productivity and glycosylation, while others had a net negative impact on productivity and glycosylation.
Keywords: Chinese hamster ovary cells (CHO); Critical quality attributes; Glycosylation; Monoclonal antibodies; Productivity.
Figures

References
-
- Agrawal V, Slivac I, Perret S, Bisson L, St-laurent G, Murad Y, Durocher Y, et al. Stable expression of chimeric heavy chain antibodies in CHO cells. Methods Mol Biol. 2012;911:287–303. - PubMed
-
- Babcock J, Antosh A. Partial replacement of chemically defined CHO media with plant-derived protein hydrolysates. Biopharm Int Suppl. 2012;23:1–6.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

