Forizymes - functionalised artificial forisomes as a platform for the production and immobilisation of single enzymes and multi-enzyme complexes
- PMID: 27502156
- PMCID: PMC4977538
- DOI: 10.1038/srep30839
Forizymes - functionalised artificial forisomes as a platform for the production and immobilisation of single enzymes and multi-enzyme complexes
Abstract
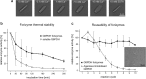
The immobilisation of enzymes plays an important role in many applications, including biosensors that require enzyme activity, stability and recyclability in order to function efficiently. Here we show that forisomes (plant-derived mechanoproteins) can be functionalised with enzymes by translational fusion, leading to the assembly of structures designated as forizymes. When forizymes are expressed in the yeast Saccharomyces cerevisiae, the enzymes are immobilised by the self-assembly of forisome subunits to form well-structured protein bodies. We used glucose-6-phosphate dehydrogenase (G6PDH) and hexokinase 2 (HXK2) as model enzymes for the one-step production and purification of catalytically active forizymes. These structures retain the typical stimulus-response reaction of the forisome and the enzyme remains active even after multiple assay cycles, which we demonstrated using G6PDH forizymes as an example. We also achieved the co-incorporation of both HXK2 and G6PDH in a single forizyme, facilitating a two-step reaction cascade that was 30% faster than the coupled reaction using the corresponding enzymes on different forizymes or in solution. Our novel forizyme immobilisation technique therefore not only combines the sensory properties of forisome proteins with the catalytic properties of enzymes but also allows the development of multi-enzyme complexes for incorporation into technical devices.
Figures




Similar articles
-
Effect of pH on the stability of hexokinase and glucose 6-phosphate dehydrogenase.Appl Biochem Biotechnol. 2002 Spring;98-100:265-72. doi: 10.1385/abab:98-100:1-9:265. Appl Biochem Biotechnol. 2002. PMID: 12018254
-
Recombinant artificial forisomes provide ample quantities of smart biomaterials for use in technical devices.Appl Microbiol Biotechnol. 2010 Oct;88(3):689-98. doi: 10.1007/s00253-010-2771-4. Epub 2010 Jul 28. Appl Microbiol Biotechnol. 2010. PMID: 20665019
-
Artificial forisomes are ideal models of forisome assembly and activity that allow the development of technical devices.Biomacromolecules. 2012 Oct 8;13(10):3076-86. doi: 10.1021/bm3008499. Epub 2012 Sep 24. Biomacromolecules. 2012. PMID: 22963540
-
Enzyme immobilisation in biocatalysis: why, what and how.Chem Soc Rev. 2013 Aug 7;42(15):6223-35. doi: 10.1039/c3cs60075k. Chem Soc Rev. 2013. PMID: 23532151 Review.
-
Immobilization and applications of glucose-6-phosphate dehydrogenase: a review.Prep Biochem Biotechnol. 2013;43(4):376-84. doi: 10.1080/10826068.2012.738274. Prep Biochem Biotechnol. 2013. PMID: 23464920 Review.
Cited by
-
Fabrication and characterization of glucose-oxidase-trehalase electrode based on nanomaterial-coated carbon paper.RSC Adv. 2023 Nov 20;13(48):33918-33928. doi: 10.1039/d3ra01554h. eCollection 2023 Nov 16. RSC Adv. 2023. PMID: 38020009 Free PMC article.
-
Efficient yeast surface-display of novel complex synthetic cellulosomes.Microb Cell Fact. 2018 Aug 7;17(1):122. doi: 10.1186/s12934-018-0971-2. Microb Cell Fact. 2018. PMID: 30086751 Free PMC article.
-
The cis-prenyltransferase protein family in Taraxacum koksaghyz.Plant J. 2025 Feb;121(3):e17233. doi: 10.1111/tpj.17233. Plant J. 2025. PMID: 39915980 Free PMC article.
-
Magnetizing Biotech-Advances in (In Vivo) Magnetic Enzyme Immobilization.Eng Life Sci. 2025 Mar 13;25(3):e70000. doi: 10.1002/elsc.70000. eCollection 2025 Mar. Eng Life Sci. 2025. PMID: 40083857 Free PMC article. Review.
-
The Ca2+ response of a smart forisome protein is dependent on polymerization.Protein Sci. 2022 Mar;31(3):602-612. doi: 10.1002/pro.4256. Epub 2021 Dec 18. Protein Sci. 2022. PMID: 34897845 Free PMC article.
References
-
- DiCosimo R., McAuliffe J., Poulose A. J. & Bohlmann G. Industrial use of immobilized enzymes. Chem Soc Rev 42, 6437–6474 (2013). - PubMed
-
- Sheldon R. A. & van Pelt S. Enzyme immobilisation in biocatalysis: why, what and how. Chem Soc Rev 42, 6223–6235 (2013). - PubMed
-
- Hanefeld U., Gardossi L. & Magner E. Understanding enzyme immobilisation. Chem Soc Rev 38, 453–468 (2009). - PubMed
-
- Cao L., Langen L. & Sheldon R. A. Immobilised enzymes: carrier-bound or carrier-free? Curr Opin Biotechnol 14, 387–394 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

