Rift Valley fever vector diversity and impact of meteorological and environmental factors on Culex pipiens dynamics in the Okavango Delta, Botswana
- PMID: 27502246
- PMCID: PMC4977755
- DOI: 10.1186/s13071-016-1712-1
Rift Valley fever vector diversity and impact of meteorological and environmental factors on Culex pipiens dynamics in the Okavango Delta, Botswana
Abstract
Background: In Northern Botswana, rural communities, livestock, wildlife and large numbers of mosquitoes cohabitate around permanent waters of the Okavango Delta. As in other regions of sub-Saharan Africa, Rift Valley Fever (RVF) virus is known to circulate in that area among wild and domestic animals. However, the diversity and composition of potential RVF mosquito vectors in that area are unknown as well as the climatic and ecological drivers susceptible to affect their population dynamics.
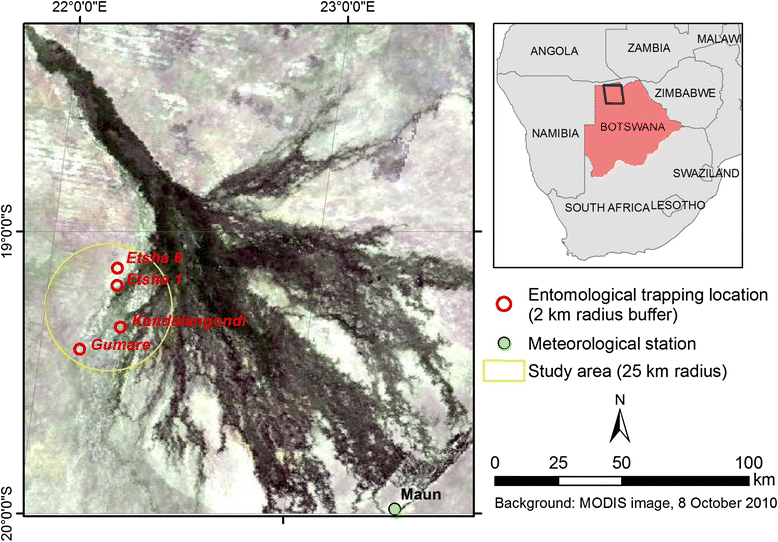
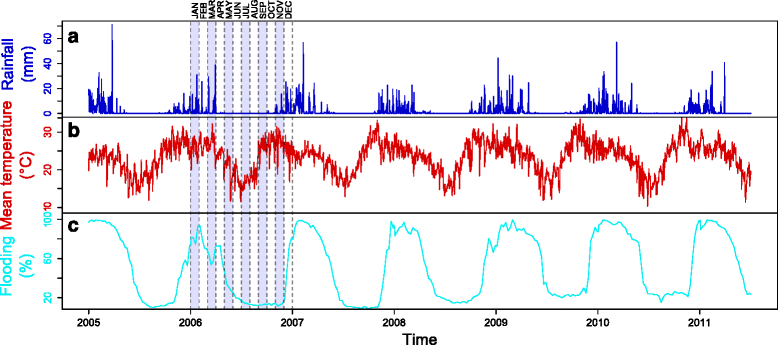
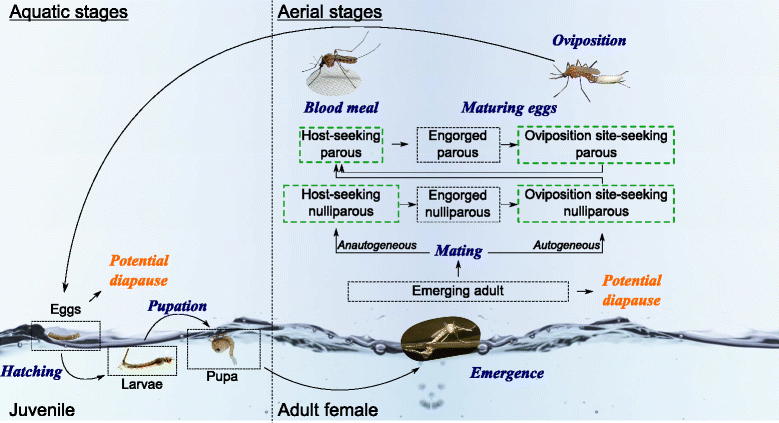
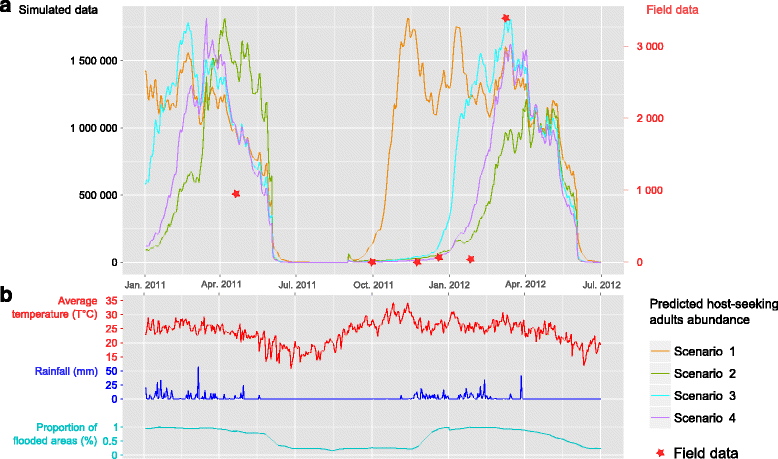
Methods: Using net traps baited with carbon dioxide, monthly mosquito catches were implemented over four sites surrounding cattle corrals at the northwestern border of the Okavango Delta between 2011 and 2012. The collected mosquito species were identified and analysed for the presence of RVF virus by molecular methods. In addition, a mechanistic model was developed to assess the qualitative influence of meteorological and environmental factors such as temperature, rainfall and flooding levels, on the population dynamics of the most abundant species detected (Culex pipiens).
Results: More than 25,000 mosquitoes from 32 different species were captured with an overabundance of Cx. pipiens (69,39 %), followed by Mansonia uniformis (20,67 %) and a very low detection of Aedes spp. (0.51 %). No RVF virus was detected in our mosquito pooled samples. The model fitted well the Cx. pipiens catching results (ρ = 0.94, P = 0.017). The spatial distribution of its abundance was well represented when using local rainfall and flooding measures (ρ = 1, P = 0.083). The global population dynamics were mainly influenced by temperature, but both rainfall and flooding presented a significant influence. The best and worst suitable periods for mosquito abundance were around March to May and June to October, respectively.
Conclusions: Our study provides the first available data on the presence of potential RVF vectors that could contribute to the maintenance and dissemination of RVF virus in the Okavango Delta. Our model allowed us to understand the dynamics of Cx. pipiens, the most abundant vector identified in this area. Potential predictions of peaks in abundance of this vector could allow the identification of the most suitable periods for disease occurrence and provide recommendations for vectorial and disease surveillance and control strategies.
Keywords: Botswana; Climatic factors; Culex pipiens; Flooding; Mosquito; Okavango Delta; Population dynamics modeling; Remote sensing; Rift Valley fever; Vector.
Figures



Similar articles
-
Distribution and abundance of key vectors of Rift Valley fever and other arboviruses in two ecologically distinct counties in Kenya.PLoS Negl Trop Dis. 2017 Feb 17;11(2):e0005341. doi: 10.1371/journal.pntd.0005341. eCollection 2017 Feb. PLoS Negl Trop Dis. 2017. PMID: 28212379 Free PMC article.
-
Effects of Irrigation and Rainfall on the Population Dynamics of Rift Valley Fever and Other Arbovirus Mosquito Vectors in the Epidemic-Prone Tana River County, Kenya.J Med Entomol. 2017 Mar 1;54(2):460-470. doi: 10.1093/jme/tjw206. J Med Entomol. 2017. PMID: 28011732 Free PMC article.
-
Biology of mosquitoes that are potential vectors of Rift Valley Fever virus in different biotopes of the central highlands of Madagascar.J Med Entomol. 2013 May;50(3):603-10. doi: 10.1603/me12069. J Med Entomol. 2013. PMID: 23802456
-
Rift Valley Fever: An Emerging Mosquito-Borne Disease.Annu Rev Entomol. 2016;61:395-415. doi: 10.1146/annurev-ento-010715-023819. Annu Rev Entomol. 2016. PMID: 26982443 Review.
-
A review of mosquitoes associated with Rift Valley fever virus in Madagascar.Am J Trop Med Hyg. 2015 Apr;92(4):722-9. doi: 10.4269/ajtmh.14-0421. Epub 2015 Mar 2. Am J Trop Med Hyg. 2015. PMID: 25732680 Free PMC article. Review.
Cited by
-
Entomological survey of the potential vectors of Rift Valley fever virus and absence of detection of the virus genome from the vectors in various niches in the southern half of the Great Rift Valley of Ethiopia.Vet Med Sci. 2022 Nov;8(6):2716-2725. doi: 10.1002/vms3.941. Epub 2022 Sep 14. Vet Med Sci. 2022. PMID: 36104829 Free PMC article.
-
Complementarity of empirical and process-based approaches to modelling mosquito population dynamics with Aedes albopictus as an example-Application to the development of an operational mapping tool of vector populations.PLoS One. 2020 Jan 17;15(1):e0227407. doi: 10.1371/journal.pone.0227407. eCollection 2020. PLoS One. 2020. PMID: 31951601 Free PMC article.
-
A historical perspective on arboviruses of public health interest in Southern Africa.Pathog Glob Health. 2024 Mar;118(2):131-159. doi: 10.1080/20477724.2023.2290375. Epub 2023 Dec 11. Pathog Glob Health. 2024. PMID: 38082563 Free PMC article. Review.
-
High seroprevalence of Rift Valley fever phlebovirus in domestic ruminants and African Buffaloes in Mozambique shows need for intensified surveillance.Infect Ecol Epidemiol. 2017 Dec 17;7(1):1416248. doi: 10.1080/20008686.2017.1416248. eCollection 2017. Infect Ecol Epidemiol. 2017. PMID: 29321827 Free PMC article.
-
Aedes species (Diptera: Culicidae) ecological and host feeding patterns in the north-eastern parts of South Africa, 2014-2018.Parasit Vectors. 2021 Jun 26;14(1):339. doi: 10.1186/s13071-021-04845-9. Parasit Vectors. 2021. PMID: 34174956 Free PMC article.
References
-
- Linthicum KJ, Anyamba A, Britch SC, Chretien JP, Erickson RL, Small J, et al. A Rift Valley fever risk surveillance system for Africa using remotely sensed data: potential for use on other continents. Vet Ital. 2007;43(3):663–74. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

