Ruxolitinib in steroid refractory graft-vs.-host disease: a case report
- PMID: 27502249
- PMCID: PMC4977623
- DOI: 10.1186/s13045-016-0298-6
Ruxolitinib in steroid refractory graft-vs.-host disease: a case report
Abstract
Background: Allogeneic hematopoietic stem cell transplantation (HSCT) is potentially curative in a variety of hematological malignancies. Graft-vs.-host disease (GvHD) remains a life-threatening complication. Standard treatment is high-dose (HD) corticosteroids. Steroid-refractory (SR) GvHD is associated with poor prognosis. At present, second-line treatment is ill-defined and includes a number of agents. Novel insights into the pathophysiology of acute GvHD (aGvHD) highlight the relevant role of the host inflammatory response governed by several kinase families, including Janus kinases (JAK)1/2. Ruxolitinib, a JAK1/2 inhibitor approved for intermediate-2/high-risk myelofibrosis, was recently employed in SR-GvHD with encouraging overall response rates. Clinical experience however remains limited.
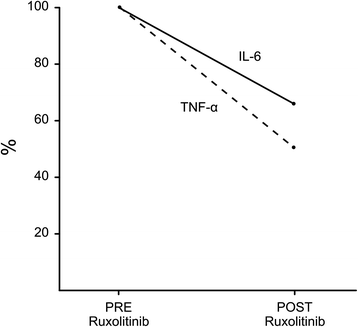
Case presentation: A 51-year-old male with refractory anemia with excess blast type-2 underwent a myeloablative allogeneic HSCT from a 9/10 HLA-matched unrelated donor after conditioning with busulfan and cyclophosphamide. GvHD prophylaxis consisted of cyclosporine, methotrexate, and thymoglobulin. CD34(+) cells/kg infused were 8.69 × 10(6) kg. On day 29, the patient developed overall grade IV aGvHD with biopsy proven stage IV gastrointestinal (GI) GvHD refractory to HD corticosteroids. Patient conditions rapidly deteriorated and became critical despite the addition of mycophenolate mofetil and budesonide. On day 33, Ruxolitinib was started, and on day 39 the patient clinical conditions gradually improved. Complete resolution of aGvHD was also confirmed by histology on day 54.
Conclusions: At 5 months from HSCT, the patient is well and in continuous hematological complete remission without flare of GvHD. Ruxolitinib was discontinued on day 156. Ruxolitinib is feasible and effective in SR-aGvHD though large prospective clinical trials are warranted.
Keywords: Allogeneic hematopoietic stem cell transplant (HSCT); Case report; Proinflammatory cytokines; Regulatory T cells (Treg); Ruxolitinib; Steroid-refractory graft-vs.-host disease (SR-GvHD).
Figures

Similar articles
-
Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey.Leukemia. 2015 Oct;29(10):2062-8. doi: 10.1038/leu.2015.212. Epub 2015 Jul 31. Leukemia. 2015. PMID: 26228813 Free PMC article.
-
Ruxolitinib in children with steroid-refractory acute graft-versus-host disease: A retrospective multicenter study of the pediatric group of SFGM-TC.Pediatr Blood Cancer. 2020 Sep;67(9):e28233. doi: 10.1002/pbc.28233. Epub 2020 Jul 2. Pediatr Blood Cancer. 2020. PMID: 32614145
-
Ruxolitinib in GvHD (RIG) study: a multicenter, randomized phase 2 trial to determine the response rate of Ruxolitinib and best available treatment (BAT) versus BAT in steroid-refractory acute graft-versus-host disease (aGvHD) (NCT02396628).BMC Cancer. 2018 Nov 19;18(1):1132. doi: 10.1186/s12885-018-5045-7. BMC Cancer. 2018. PMID: 30453910 Free PMC article. Clinical Trial.
-
Refractory acute graft-versus-host disease: a new working definition beyond corticosteroid refractoriness.Blood. 2020 Oct 22;136(17):1903-1906. doi: 10.1182/blood.2020007336. Blood. 2020. PMID: 32756949 Review.
-
Ruxolitinib: a potential treatment for corticosteroid refractory acute graft-versus-host disease.Expert Opin Investig Drugs. 2020 May;29(5):423-427. doi: 10.1080/13543784.2020.1757069. Epub 2020 Apr 19. Expert Opin Investig Drugs. 2020. PMID: 32293938 Review.
Cited by
-
Attenuated Novel SARS Coronavirus 2 Infection in an Allogeneic Hematopoietic Stem Cell Transplant Patient on Ruxolitinib.Clin Lymphoma Myeloma Leuk. 2020 Nov;20(11):720-723. doi: 10.1016/j.clml.2020.06.014. Epub 2020 Jun 25. Clin Lymphoma Myeloma Leuk. 2020. PMID: 32727701 Free PMC article.
-
FDA Approval Summary: Ruxolitinib for Treatment of Steroid-Refractory Acute Graft-Versus-Host Disease.Oncologist. 2020 Feb;25(2):e328-e334. doi: 10.1634/theoncologist.2019-0627. Epub 2019 Oct 22. Oncologist. 2020. PMID: 32043777 Free PMC article.
-
Cutaneous Graft-Versus-Host Disease: Diagnosis and Treatment.Am J Clin Dermatol. 2018 Feb;19(1):33-50. doi: 10.1007/s40257-017-0306-9. Am J Clin Dermatol. 2018. PMID: 28656563 Free PMC article. Review.
-
Advance in Targeted Immunotherapy for Graft-Versus-Host Disease.Front Immunol. 2018 May 16;9:1087. doi: 10.3389/fimmu.2018.01087. eCollection 2018. Front Immunol. 2018. PMID: 29868032 Free PMC article. Review.
-
Cancer Immunotherapy, Part 2: Efficacy, Safety, and Other Clinical Considerations.P T. 2017 Jul;42(7):452-463. P T. 2017. PMID: 28674473 Free PMC article.
References
-
- Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, Litzow MR, Nieto Y, Savani BN, Schriber JR, Shaughnessy PJ, Wall DA, Carpenter PA. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18(8):1150–1163. doi: 10.1016/j.bbmt.2012.04.005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

