Olfactory perception of chemically diverse molecules
- PMID: 27502425
- PMCID: PMC4977894
- DOI: 10.1186/s12868-016-0287-2
Olfactory perception of chemically diverse molecules
Abstract
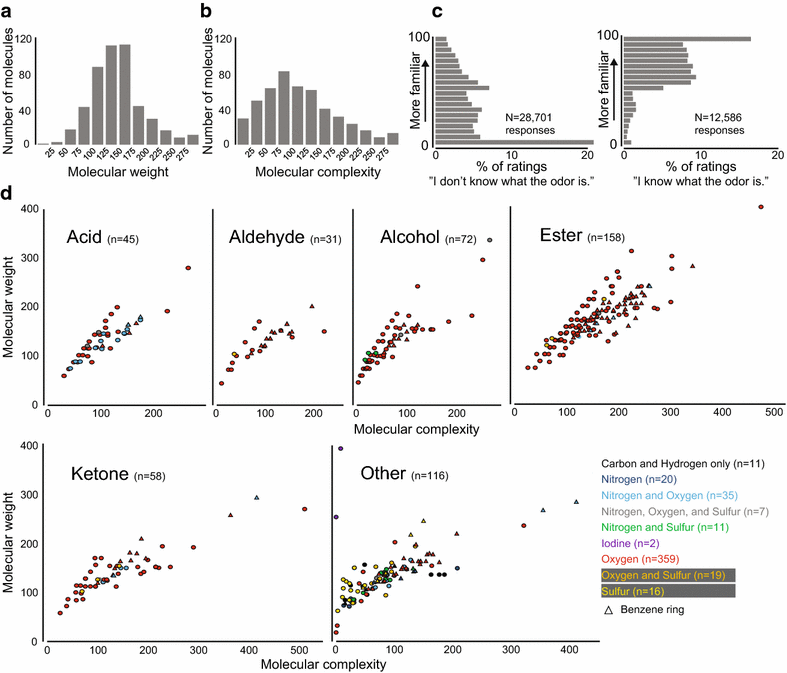
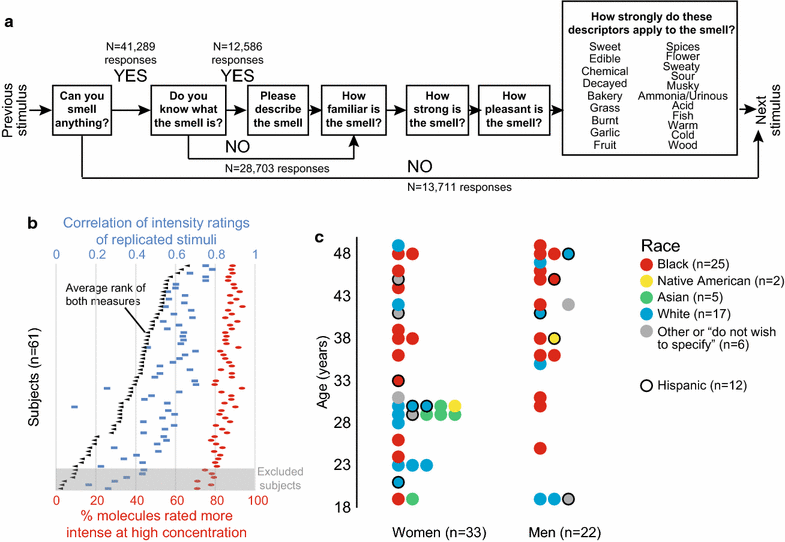
Background: Understanding the relationship between a stimulus and how it is perceived reveals fundamental principles about the mechanisms of sensory perception. While this stimulus-percept problem is mostly understood for color vision and tone perception, it is not currently possible to predict how a given molecule smells. While there has been some progress in predicting the pleasantness and intensity of an odorant, perceptual data for a larger number of diverse molecules are needed to improve current predictions. Towards this goal, we tested the olfactory perception of 480 structurally and perceptually diverse molecules at two concentrations using a panel of 55 healthy human subjects.
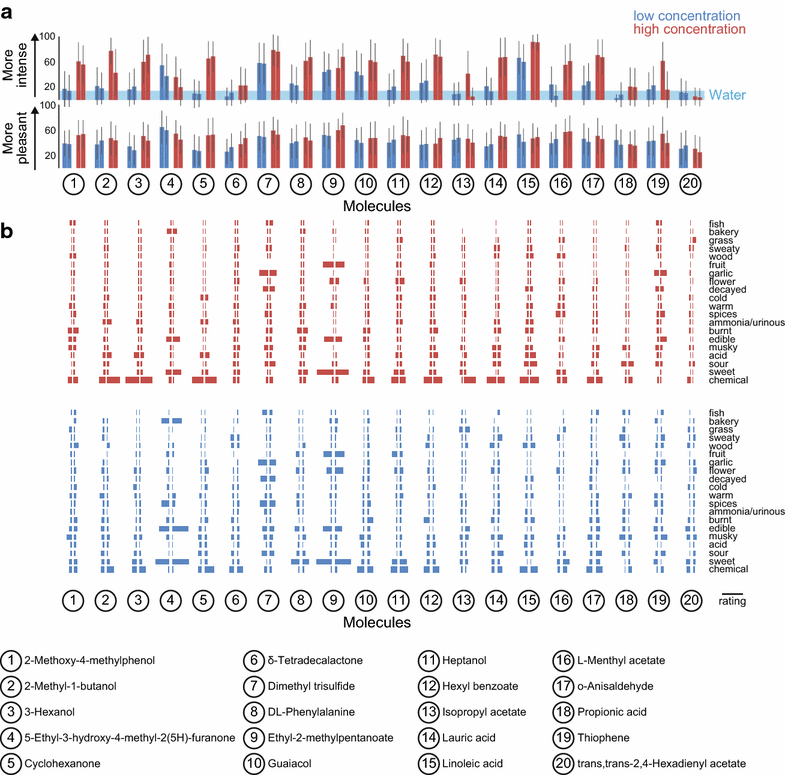
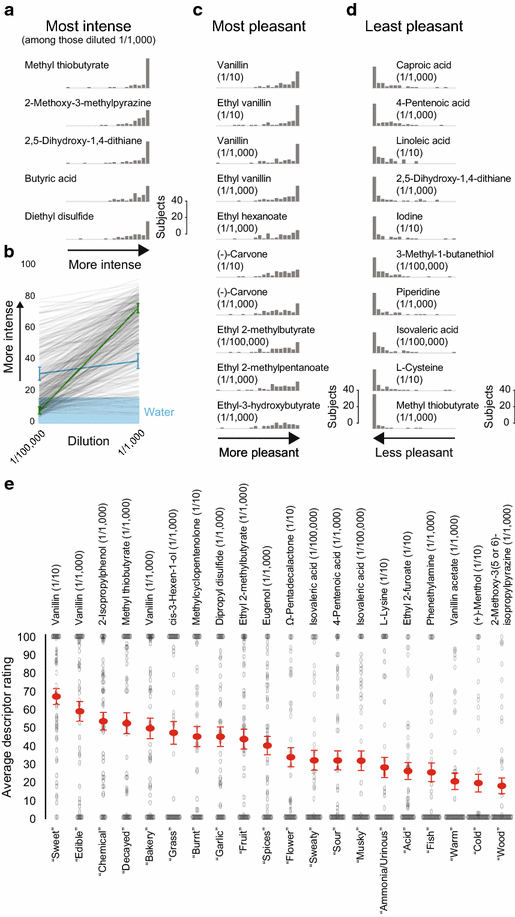
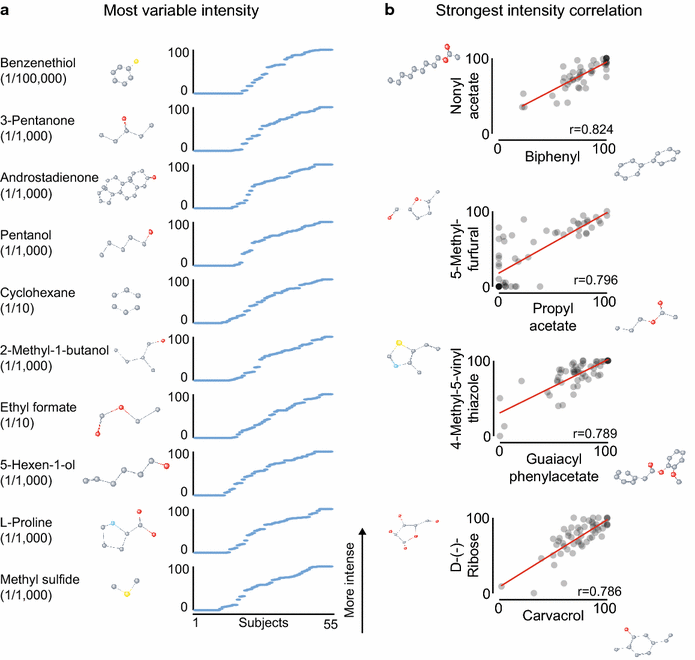
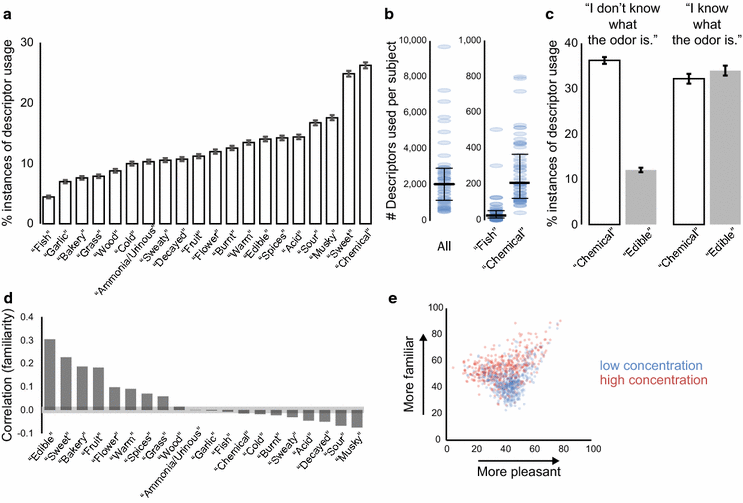
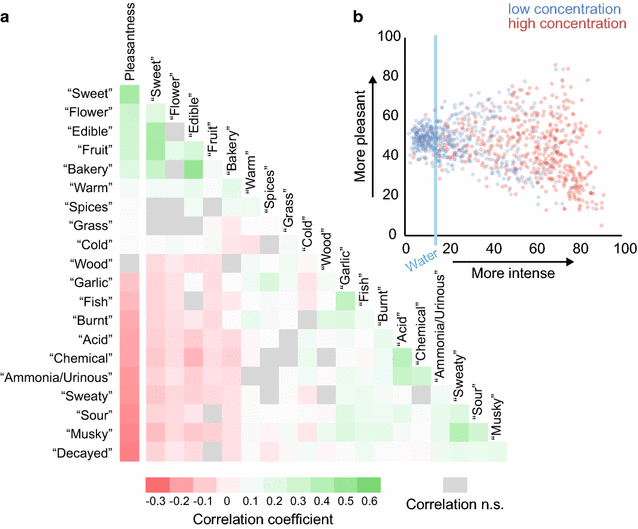
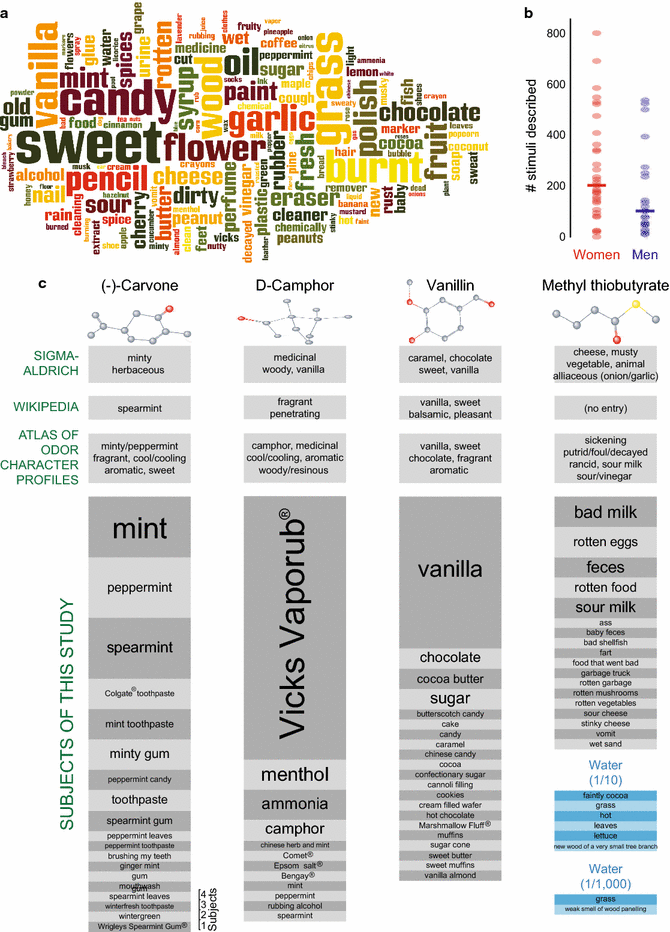
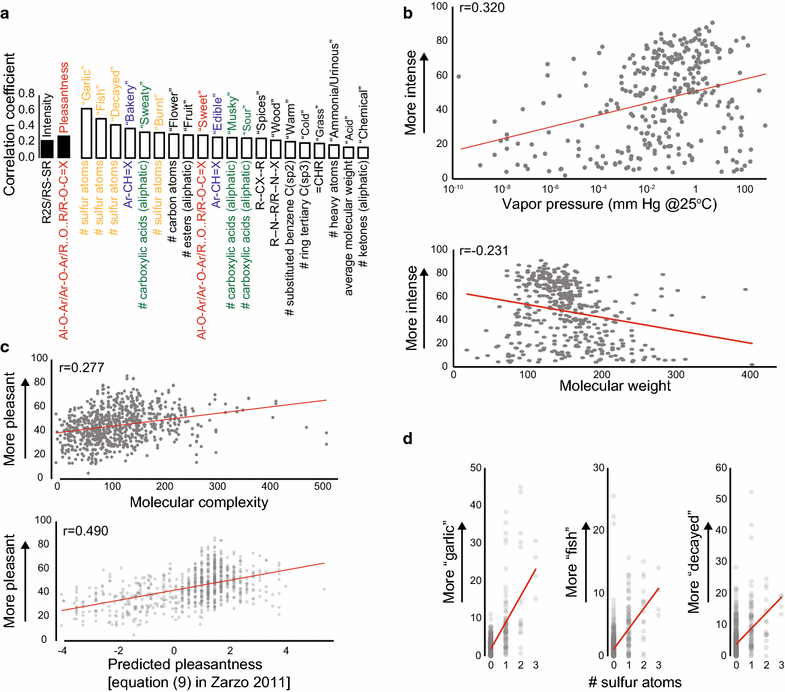
Results: For each stimulus, we collected data on perceived intensity, pleasantness, and familiarity. In addition, subjects were asked to apply 20 semantic odor quality descriptors to these stimuli, and were offered the option to describe the smell in their own words. Using this dataset, we replicated several previous correlations between molecular features of the stimulus and olfactory perception. The number of sulfur atoms in a molecule was correlated with the odor quality descriptors "garlic," "fish," and "decayed," and large and structurally complex molecules were perceived to be more pleasant. We discovered a number of correlations in intensity perception between molecules. We show that familiarity had a strong effect on the ability of subjects to describe a smell. Many subjects used commercial products to describe familiar odorants, highlighting the role of prior experience in verbal reports of olfactory perception. Nonspecific descriptors like "chemical" were applied frequently to unfamiliar odorants, and unfamiliar odorants were generally rated as neither pleasant nor unpleasant.
Conclusions: We present a very large psychophysical dataset and use this to correlate molecular features of a stimulus to olfactory percept. Our work reveals robust correlations between molecular features and perceptual qualities, and highlights the dominant role of familiarity and experience in assigning verbal descriptors to odorants.
Keywords: Cheminformatics; Odor descriptors; Olfaction; Perceptual familiarity; Perceptual variability; Psychophysics; Structure-odor-relationship.
Figures
References
-
- Boelens H. Structure activity relationships in chemoreception by human olfaction. Trends Pharmacol Sci. 1983;4(10):421–426. doi: 10.1016/0165-6147(83)90475-3. - DOI
-
- Edwards PA, Jurs PC. Correlation of odor intensities with structural properties of odorants. Chem Senses. 1989;14(2):281–291. doi: 10.1093/chemse/14.2.281. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

