A Simplified, Langendorff-Free Method for Concomitant Isolation of Viable Cardiac Myocytes and Nonmyocytes From the Adult Mouse Heart
- PMID: 27502479
- PMCID: PMC5965670
- DOI: 10.1161/CIRCRESAHA.116.309202
A Simplified, Langendorff-Free Method for Concomitant Isolation of Viable Cardiac Myocytes and Nonmyocytes From the Adult Mouse Heart
Abstract
Rationale: Cardiovascular disease represents a global pandemic. The advent of and recent advances in mouse genomics, epigenomics, and transgenics offer ever-greater potential for powerful avenues of research. However, progress is often constrained by unique complexities associated with the isolation of viable myocytes from the adult mouse heart. Current protocols rely on retrograde aortic perfusion using specialized Langendorff apparatus, which poses considerable logistical and technical barriers to researchers and demands extensive training investment.
Objective: To identify and optimize a convenient, alternative approach, allowing the robust isolation and culture of adult mouse cardiac myocytes using only common surgical and laboratory equipment.
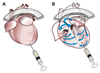
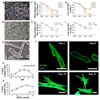
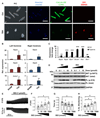
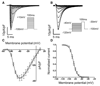
Methods and results: Cardiac myocytes were isolated with yields comparable to those in published Langendorff-based methods, using direct needle perfusion of the LV ex vivo and without requirement for heparin injection. Isolated myocytes can be cultured antibiotic free, with retained organized contractile and mitochondrial morphology, transcriptional signatures, calcium handling, responses to hypoxia, neurohormonal stimulation, and electric pacing, and are amenable to patch clamp and adenoviral gene transfer techniques. Furthermore, the methodology permits concurrent isolation, separation, and coculture of myocyte and nonmyocyte cardiac populations.
Conclusions: We present a novel, simplified method, demonstrating concomitant isolation of viable cardiac myocytes and nonmyocytes from the same adult mouse heart. We anticipate that this new approach will expand and accelerate innovative research in the field of cardiac biology.
Keywords: Langendorff-free; cardiac fibroblasts; cardiomyocytes; cardiovascular disease; coculture; mouse models; single-cell isolation.
© 2016 American Heart Association, Inc.
Figures


Comment in
-
With or Without Langendorff: A New Method for Adult Myocyte Isolation to Be Tested With Time.Circ Res. 2016 Sep 30;119(8):888-90. doi: 10.1161/CIRCRESAHA.116.309734. Circ Res. 2016. PMID: 27688302 Free PMC article. No abstract available.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

